News & Events
Cambridge Antibody Technology Breaks Old Myths on Rejection

You see new hope in kidney transplantation when you look at the advances from Cambridge Antibody Technology. Monoclonal antibodies now help you target harmful immune responses with more precision. New detection techniques, such as Luminex and C4d assays, give doctors better tools to protect your transplanted kidney.
- Ten years after a transplant, antibody-mediated rejection affects:
- 17% of young recipients
- 15% of middle-aged recipients
- 12% of elderly recipients
| Detection Technique | Impact on Clinical Outcomes |
|---|---|
| Luminex Technique | Finds more risks by detecting antibodies earlier and more accurately. |
| C4d Assay | Shows a strong link between certain antibodies and kidney graft survival. |
| C1q Assay | Helps identify dangerous antibodies, which may improve your treatment. |
Key Takeaways
- Monoclonal antibodies improve kidney transplant outcomes by targeting harmful immune responses more precisely.
- New detection techniques like Luminex and C4d assays help doctors identify risks earlier, enhancing patient care.
- Understanding common myths about antibody-mediated rejection empowers patients to make informed health decisions.
- Advanced therapeutic strategies, including combination therapies, significantly increase graft survival rates after kidney transplants.
- Ongoing research and innovations in antibody detection and treatment offer hope for better management of kidney transplantation.
Antibody-Mediated Rejection
What Is Antibody-Mediated Rejection
You may hear doctors talk about antibody-mediated rejection when you learn about organ transplants. This process happens when your immune system makes antibodies that attack the transplanted organ. These antibodies often target donor human leukocyte antigen, or HLA, on the new kidney. When this occurs, you face a risk of losing the transplanted organ.
Doctors use a mix of tests to diagnose antibody-mediated rejection. They look for signs of damage in the kidney tissue and check for donor specific antibodies in your blood. Sometimes, you develop de novo donor-specific antibodies after the transplant. These new antibodies can lead to acute antibody-mediated rejection or even chronic antibody-mediated rejection over time. You may need special treatments, such as immunosuppression, to help control this process.
Antibody-mediated rejection is complex. You need both lab tests and tissue samples to confirm it. This approach helps doctors find the best way to protect your kidney.
Common Myths
Many people believe myths about antibody-mediated rejection. These myths can make it harder for you to understand your treatment options. Here are some common misconceptions:
-
Myth: Only T cells cause rejection.
You might think T cells are the only problem, but antibodies play a big role. Antibody-mediated rejection can happen even when T cells are under control. -
Myth: Antibody-mediated rejection only happens right after transplant.
In reality, you can develop antibody-mediated rejection years later. De novo donor-specific antibodies may appear long after surgery. -
Myth: All antibodies are harmful.
Not every antibody leads to rejection. Doctors focus on donor specific antibodies that target the transplanted organ. -
Myth: Acute antibody-mediated rejection is always easy to spot.
Sometimes, you may not feel sick at first. Doctors rely on tests to find early signs of antibody-mediated rejection. -
Myth: Chronic antibody-mediated rejection cannot be managed.
New treatments and better detection help you fight chronic antibody-mediated rejection and protect your kidney for longer.
Understanding these facts helps you work with your care team. You can ask better questions and make informed choices about your health.
Cambridge Antibody Technology

Monoclonal Antibody Development
You see major changes in how doctors approach organ transplantation because of cambridge antibody technology. This company leads the way in developing therapeutic antibodies that help you fight rejection and improve your chances of a successful transplant. You benefit from their work on fully human antibodies, which are safer and more effective for your treatment.
Cambridge antibody technology made history with several milestones. You can see their impact in the table below:
| Milestone | Description |
|---|---|
| Development of Phage Display Technology | Sir Greg Winter pioneered this technology, which became the foundation for creating therapeutic antibodies. |
| Discovery of Humira | Adalimumab (Humira) was the first fully human antibody blockbuster drug, changing the treatment of autoimmune diseases. |
| Development of Belimumab | Belimumab (Benlysta) became the first new drug for systemic lupus in over 50 years. |
| Millennium Product Status | In 1999, CAT’s Library product and ProAb received Millennium Product status for innovation in biotechnology. |
You gain access to therapeutic antibodies like Humira and Belimumab because of these breakthroughs. Cambridge antibody technology also developed anti-CD25 Daclizumab, which the FDA approved in 1997 for transplant rejection treatment. You see better outcomes because fully human antibodies reduce side effects and improve safety in your treatment.
The process of developing monoclonal antibodies is not easy. You face challenges such as high attrition rates, resource-intensive production, and the need for careful safety assessments. Many steps in manufacturing affect the properties of each antibody, which can change how well your treatment works.
You notice that hybridoma technology started the field, but humanization of antibodies made them safer for you. Cambridge antibody technology uses cell-based systems to produce therapeutic antibodies, which can be costly and complex. Despite these challenges, you benefit from more effective and safer treatment options.
Monoclonal antibody therapies give you better results than traditional immunosuppressive drugs. You experience fewer side effects, such as rash or infusion reactions, and you tolerate these treatments better. Fully human antibodies offer a better safety profile, especially for women and elderly patients. You see improved survival rates and less toxicity compared to older treatments.
Anti-HLA Antibody Detection
You rely on accurate detection of anti-HLA antibodies to guide your treatment and protect your transplanted organ. Cambridge antibody technology transformed this process with advanced assays. You see the difference when doctors use Luminex-based tests instead of older CDC assays.
- Luminex technology detects low levels of HLA antibodies, which helps you get a more detailed analysis of your antibody profile.
- The LSAB assay uses fluorescence to find specific antibodies with high accuracy and sensitivity.
- Luminex single-antigen bead assays show antibody specificity at the allele level, giving you better information for your treatment.
You notice that traditional CDC assays often miss low-level antibodies and can produce false negatives. This can affect your treatment decisions and put your transplant at risk. Cambridge antibody technology’s Luminex-based assays provide higher sensitivity and specificity, so you get more reliable results.
The main barriers to using new detection technologies include assay sensitivity, lack of standardization, and challenges in interpreting results. Some tests may give false positives or negatives, and different labs may get different results. You need harmonized measurement techniques to make sure your treatment decisions are based on accurate data.
| Barrier Type | Description |
|---|---|
| Assay Sensitivity | New assays may produce false positives from denatured antigens and false negatives due to low antibody titers. |
| Lack of Standardization | Variability in assay results due to non-harmonized measurement techniques across laboratories. |
| Interpretation Challenges | Difficulty in accurately estimating antibody levels without harmonization leads to non-comparable results. |
You see that some transplant centers still use older serological typing methods. This gap in adoption can affect your treatment and outcomes. Cambridge antibody technology’s innovations help you get better care by improving the accuracy and sensitivity of antibody detection.
You benefit from these advancements because doctors can tailor your treatment more precisely. You get better protection for your transplanted organ, and you avoid unnecessary risks. Cambridge antibody technology continues to lead the way in developing therapeutic antibodies and detection tools that improve your health and quality of life.
Advancements in Antibody-Mediated Treatments

Therapeutic Antibodies
You now have access to new antibody-mediated rejection treatment strategies that offer hope for better outcomes. Therapeutic antibodies have changed how you manage both acute antibody-mediated rejection and chronic antibody-mediated rejection. These therapies target the harmful donor-specific antibodies that threaten your transplanted organ.
Recent advancements in therapeutic antibodies include new ways to target cells that produce these antibodies. You benefit from a multifaceted approach that goes beyond traditional drugs. Here are some of the latest developments:
- Scientists now use monoclonal antibodies that target CD38. This approach helps remove plasma cells and innate effector cells that drive antibody-mediated rejection.
- The CD38 antibody felzartamab has shown promise in phase 2 trials. You may see this as a future option for treating antibody-mediated rejection.
- These new therapies help you by reducing the number of donor-specific antibodies and lowering the risk of acute antibody-mediated rejection.
You also see a range of treatment strategies that work together to control antibody-mediated rejection. Each therapy targets a different part of the immune response. The table below shows how these treatments work:
| Therapeutic Modality | Mechanism of Action |
|---|---|
| Plasmapheresis/Immunoadsorption | Removes circulating donor-specific antibodies but does not stop antibody production. |
| Intravenous Immunoglobulins (IVIG) | Neutralizes donor-specific antibodies and blocks complement activation. |
| Rituximab | Depletes B cells, stopping them from becoming plasma cells that make antibodies. |
| Bortezomib | Causes plasma cells to die, reducing antibody production. |
| Antithymocyte Globulin | Causes B cell death and reduces helper T cells, controlling the antibody-mediated response. |
| Eculizumab | Blocks complement activation, lowering the risk of acute antibody-mediated rejection. |
You may receive a combination of these therapies. Doctors often use plasmapheresis and IVIG together. This combination removes harmful donor-specific antibodies and helps your body accept the transplanted organ. Clinical studies show that patients who receive both plasmapheresis and IVIG have higher graft survival rates and better patient survival compared to those who receive plasmapheresis alone.
| Treatment Group | Graft Survival Rate | Patient Survival Rate | Infectious Complications |
|---|---|---|---|
| PP (N=13) | 46.2% | 76.9% | Similar |
| PP + IVIg (N=11) | 90.9% | 100% | Similar |
You see that adding IVIG to plasmapheresis nearly doubles the graft survival rate. This combination also improves your chances of living longer after a kidney transplant. Doctors may also add rituximab to further reduce the risk of antibody-mediated rejection. In some cases, you may receive bortezomib or eculizumab if other treatments do not work.
You benefit from these treatment strategies because they target both the donor-specific antibodies and the cells that produce them. This approach helps you manage both acute antibody-mediated rejection and chronic antibody-mediated rejection. You get a better chance to keep your transplanted organ healthy for years.
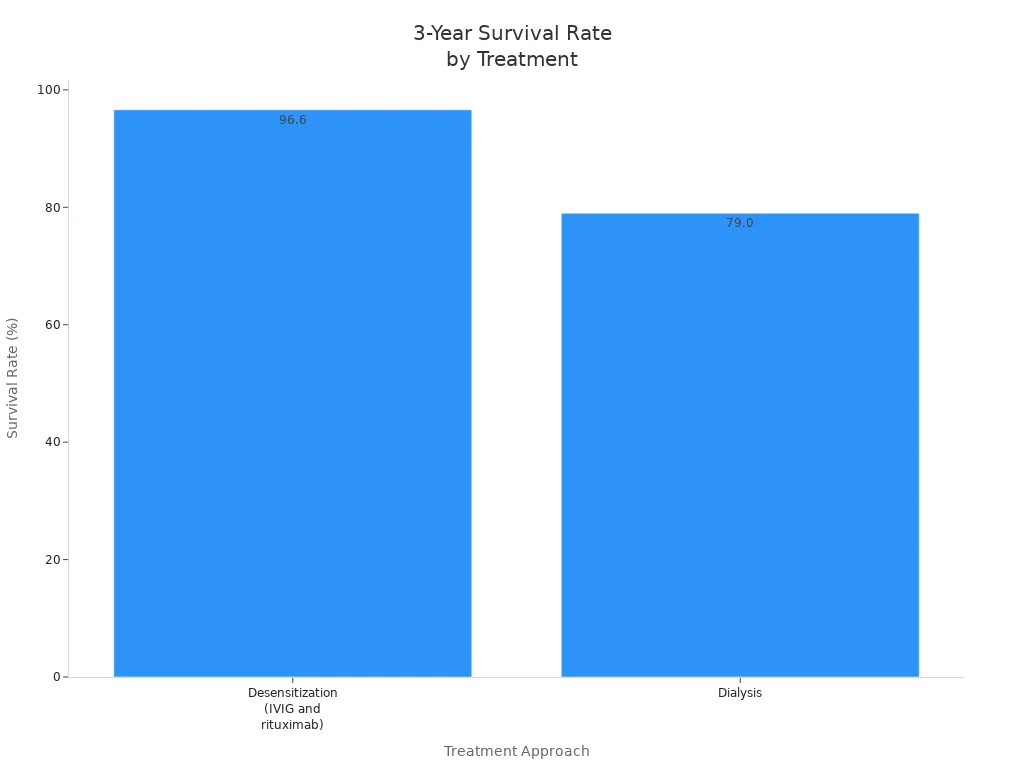
Long-term studies show that advanced therapeutic antibodies improve survival rates. Patients who receive desensitization with IVIG and rituximab have a 96.6% three-year survival rate. This is much higher than the 79% survival rate for patients who stay on dialysis. You also see cost savings with these treatment strategies.
Despite these advances, antibody-mediated rejection remains a challenge. You still face risks of chronic rejection, and doctors continue to search for better ways to manage both acute and chronic antibody-mediated rejection. Ongoing research into new antibodies and combination therapies gives you hope for even better outcomes in the future.
Improved Diagnosis
You now have access to improved diagnostic tools that help doctors detect antibody-mediated rejection earlier and more accurately. Early detection is key to successful treatment and long-term graft survival. New assays and tests give you and your care team more information about your risk of antibody-mediated rejection.
The table below shows some of the most important diagnostic tools:
| Diagnostic Tool | Description | Impact on AMR Detection and Management |
|---|---|---|
| Advanced Assays for DSA | Identify donor-specific anti-HLA antibodies | Crucial for early detection and risk stratification, improving long-term survival |
| Single-Antigen Bead (SAB) Assay | Finds all antibody specificities in complex sera | Shows antibody strength and helps diagnose humoral rejection |
| C1q Assay | Detects antibodies that bind and fix C1q | Helps doctors know which patients may face aggressive inflammation and graft damage |
You benefit from these tools because they help doctors find donor-specific antibodies before you have symptoms. The single-antigen bead assay gives detailed information about the strength and type of antibodies in your blood. The C1q assay shows if your antibodies can cause more damage, which helps your doctor choose the best treatment strategies.
Doctors use these tests to guide your treatment. If you have high levels of donor-specific antibodies, your care team may start treatment before you develop signs of acute antibody-mediated rejection. This proactive approach helps you avoid serious complications and improves your chances of keeping your transplanted organ.
You also see improvements in how doctors monitor your response to treatment. Repeat testing shows if your donor-specific antibodies decrease after therapy. If your antibody levels stay high, your doctor may adjust your treatment strategies to better protect your kidney.
Early and accurate diagnosis of antibody-mediated rejection gives you the best chance for a healthy transplant. You and your care team can make informed decisions and act quickly to prevent damage.
You now have more options and better information than ever before. New antibody-mediated rejection treatment strategies and diagnostic tools give you hope for longer, healthier lives after transplantation.
Kidney Transplantation Impact
Clinical Outcomes
You see many changes in kidney transplantation because of new antibody-mediated rejection strategies. Doctors now use advanced antibody detection and treatment methods to help you keep your transplanted kidney healthy. You may think that these innovations always lead to better results. However, the long-term success rates of kidney transplantation have not improved as much as you might expect. Each year after the first year, about 4% of kidney transplants still fail. This means that even with better antibody-mediated rejection detection and treatment, you face ongoing risks.
You need to understand how antibody-mediated rejection affects your kidney transplantation journey. Doctors focus on finding donor-specific antibodies early. They use new antibody tests to guide your treatment. These tests help you avoid sudden loss of your transplanted kidney. You benefit from therapies that target antibody-mediated rejection, but you must stay alert for signs of chronic rejection.
Case Studies
You can look at real-world examples to see how antibody-mediated rejection treatment changes outcomes in kidney transplantation. In one recent study, doctors followed eight people who received advanced antibody-mediated therapies after kidney transplantation. The results show how these new approaches help you:
| Outcome Description | Statistic |
|---|---|
| Participants alive at 5 years post-transplant | 7 of 8 |
| Participants with well-functioning grafts at 5 years | 6 of 7 |
| Mean eGFR at 5 years among alive patients | 42.3 mL/min/1.73 m² (SD = ±22) |
| Major infections over 5 years | 5 participants |
| Episodes of acute AMR beyond the first 30 days | 0 participants |
You notice that most patients stayed alive and kept their kidney working well after five years. No one had acute antibody-mediated rejection after the first month. Doctors used donor-specific antibody tests to guide treatment and prevent rejection. Some patients did face infections, so you must work closely with your care team to manage risks.
You can see that antibody-mediated rejection treatment and early detection give you a better chance to keep your transplanted kidney healthy. You need to follow your treatment plan and attend regular check-ups to catch any problems early.
Breaking Old Myths
Evidence-Based Shifts
You may have heard many outdated beliefs about antibody-mediated rejection in kidney transplantation. In the past, doctors often thought that only T cells caused rejection or that antibody-mediated rejection could not be managed. Today, you see a new approach based on strong evidence. Researchers have learned much more about the pathophysiology of antibody-mediated rejection. You now benefit from better detection of anti-HLA antibody production and improved understanding of B cells and plasma cells.
Doctors use new diagnostic tools and more aggressive treatment strategies. Early intervention with increased immunosuppression leads to faster resolution of antibody-mediated rejection episodes. You see this shift in clinical practice, where doctors act quickly when they detect donor-specific antibodies. The table below shows how evidence has changed the way doctors treat and diagnose antibody-mediated rejection in kidney transplantation:
| Evidence Type | Description |
|---|---|
| Treatment Variability | Doctors now choose treatments based on severity, from IV steroids to aggressive combinations. |
| Outcomes | 62.5% of patients experience complete resolution of antibody-mediated rejection. |
| Recommendations | Early and aggressive immunosuppressive treatment is now recommended for better outcomes. |
| Diagnostic Criteria | Improved criteria help doctors detect antibody-mediated rejection more accurately. |
| Importance of Early Intervention | Early action leads to quicker recovery and better kidney transplantation results. |
You see that these changes help you keep your transplanted kidney healthier for longer. Doctors now use surveillance protocols, such as donor-derived cell-free DNA, to catch antibody-mediated rejection early.
Future Directions
You can expect even more progress in the management of antibody-mediated rejection. Ongoing research focuses on new antibody therapies and better ways to monitor your kidney transplantation. Scientists are studying clazakizumab, a humanized monoclonal antibody that targets interleukin-6. Early studies show promise for treating antibody-mediated rejection in kidney transplantation. The IMAGINE trial is testing interleukin-6 blockade for chronic active antibody-mediated rejection.
Researchers are also developing new desensitization protocols. These help highly sensitized and ABO-incompatible patients receive kidney transplantation with better long-term survival. Advancements in HLA antibody detection have reduced the risk of immediate graft loss. You may soon see new diagnostic techniques that make it easier to identify antibody-mediated rejection before symptoms appear.
You benefit from these future directions because they offer hope for improved treatment and longer-lasting kidney transplantation. Scientists continue to explore the immunobiology of antibody-mediated rejection, aiming for therapies that protect your kidney and improve your quality of life.
You see how Cambridge Antibody Technology has changed the way you approach kidney transplantation. Their work gives you better tools and treatments for antibody-mediated rejection. Innovation remains vital for kidney transplantation and patient care. Studies show that new technology, education, and screening improve outcomes:
| Initiative/Study | Findings |
|---|---|
| UK initiative | Technology and culture changes reduce inequity and improve patient experience. |
| Norway trial | Education boosts knowledge and self-efficacy for kidney transplantation. |
You can expect new therapies and digital health tools to shape the future of kidney transplantation.
FAQ
What is antibody-mediated rejection (AMR)?
You experience AMR when your immune system makes antibodies that attack your transplanted organ. These antibodies target donor cells, which can damage your new kidney. Doctors use special tests to find and treat AMR early.
How do monoclonal antibodies help prevent rejection?
You receive monoclonal antibodies to block harmful immune responses. These medicines target specific cells or proteins that cause rejection. You get better protection for your transplanted kidney and fewer side effects than with older drugs.
Why is early detection of anti-HLA antibodies important?
You need early detection to catch rejection before symptoms appear. Doctors use advanced tests, like Luminex, to find these antibodies. Early action helps you keep your transplanted kidney healthy and improves your long-term outcome.
Can you manage chronic antibody-mediated rejection?
You can manage chronic AMR with new therapies and regular monitoring. Treatments like IVIG, plasmapheresis, and monoclonal antibodies help control the immune response. You work with your care team to adjust your treatment plan as needed.
What are the risks of new antibody therapies?
You may face risks like infections or allergic reactions. Doctors monitor you closely to catch problems early. Most patients tolerate these therapies well, and you often see better results than with older treatments.

