News & Events
How to Analyze Binding Cap Interactions in Antibody Development

You analyze binding cap interactions in antibody development by focusing on how the binding cap helps the antibody recognize its target. This process matters in research because the cap affects how well the antibody binds to molecules like mRNA. When you study these interactions, you learn how to improve antibody design for better medical treatments.
Key Takeaways
- Understand the binding cap’s role in antibody development. It helps antibodies recognize and attach to their targets, improving their effectiveness in research and therapy.
- Utilize structural and biochemical methods to analyze binding cap interactions. Techniques like X-ray crystallography and ELISA provide valuable insights into how antibodies bind to their targets.
- Leverage computational tools to predict binding interactions. Software like ZDOCK and UCSF Chimera can help identify strong antibody candidates quickly, enhancing your research efficiency.
- Optimize antibody candidates by modifying the binding cap. Small changes can significantly increase binding strength, leading to better therapeutic outcomes.
- Analyze binding kinetics to evaluate antibody performance. Measuring parameters like association and dissociation rates helps you select the best candidates for further testing.
Binding Cap Basics
What Is a Binding Cap?
You can think of a binding cap as a special region on an antibody or protein that helps it attach to its target. This cap often sits at the tip of the antibody, where it meets other molecules. Scientists have found that these caps show unique features:
- Antibody-bound peptides often have flexible shapes, with most showing irregular or loop structures.
- The same peptide can look different when it binds to different antibodies.
- These interfaces usually have less buried surface area and fewer hydrogen bonds than those seen in larger protein complexes.
- Antibody-peptide interfaces show higher binding energy per area because they have more hydrophobic residues and better shape matching.
- Over half of antibody-bound peptides do not have regular shapes, while some form turns or short helices.
Antibodies use certain amino acids, like tyrosine, serine, and glycine, more often in their binding caps than other proteins do. This pattern helps antibodies recognize many targets. Some single-domain antibodies also use arginine, which can increase how tightly they bind.
Role in Antibody and mRNA Interactions
The binding cap plays a key role in how antibodies and proteins interact with both proteins and nucleic acids. When you study capped mRNA, you see that the cap structure at the end of the RNA helps guide proteins to the right spot. Some proteins, like AGO2, use conserved amino acids to recognize the cap. If you change these amino acids, the protein cannot bind the cap, and this stops it from controlling gene expression.
| Study | Findings |
|---|---|
| Kiriakidou et al (2007) | Found that certain amino acids in AGO2 are needed to bind the cap. Mutations stop cap binding and block gene control. |
| Djuranovic et al (2010) | Showed that dmAGO1 binds better to capped mRNA when miRNAs are present, hinting at a special cap-binding site. |
| Current Study | Did not find a specific cap interaction in hAGO2, suggesting some binding may be non-specific. |
You can see that the cap structure helps proteins tell the difference between capped mRNA and uncapped mRNA. This selectivity is important for controlling which RNA gets used in the cell. When you analyze these interactions, you learn how antibodies and proteins find their targets and avoid the wrong ones.
Importance in Antibody Research
Impact on Specificity and Affinity
You need to understand how binding caps shape antibody specificity and avidity. These features help you tell how well an antibody can find and stick to its target. High-throughput ELISA assays let you test many antibodies at once. You can see which ones bind best to a certain antigen. Capture ELISA and competitive ELISA help you measure both specificity and cross-reactivity. This means you can pick antibodies that work well for your research.
A quantitative peptide immunoprecipitation assay shows that the strength of antigen-antibody interactions can change a lot. The apparent dissociation constants for these interactions vary, which means binding caps play a big part in both specificity and affinity. You need to measure these differences to get reliable results, especially in experiments like chromatin immunoprecipitation. When you modify the binding cap, you can boost the binding capacity. For example, N-terminally extended peptides can increase binding by more than a thousand times in some tests. This shows that small changes in the cap can make a big difference in how well an antibody works.
Therapeutic and Diagnostic Relevance
You use antibody specificity and avidity to improve both therapeutics and diagnostics. AI platforms now help you find monoclonal antibodies with high affinity. These tools let you screen many candidates quickly, so you can choose the best ones for your needs. AI models also help you design vaccines by mapping out the best regions for immune response. This makes your vaccine research faster and more accurate.
Predictive models help you optimize antibodies for binding, solubility, and safety. This is key for clinical success. When you combine AI with high-throughput platforms, you speed up drug discovery. You can find high-affinity binders much faster, which helps you bring new therapeutics to patients sooner.
You see that careful analysis of binding cap interactions leads to better antibody candidates. This improves outcomes in both research and patient care.
Binding Cap and mRNA Interactions
Capped mRNA Recognition
You see that capped mRNA plays a vital role in how cells control gene expression. The cap structure at the end of mRNA helps protect the molecule from decay. Cap-binding proteins attach to this cap and block enzymes that would remove it. This action keeps the mRNA stable and ready for translation. When the cap is missing, decapping enzymes break the triphosphate bridge, and the mRNA quickly decays. You can observe these mechanisms in many studies. Antibodies show a strong preference for inhibiting the translation of natural reovirus mRNA. This effect highlights the importance of the cap structure in mRNA function.
- Cap-binding proteins increase mRNA stability by blocking decay enzymes.
- Decapping enzymes remove the cap, leading to mRNA breakdown.
- Antibodies can inhibit translation by targeting the cap structure.
You can use these facts to understand why the enrichment of capped mRNA is important in research. The binding cap helps you study the binding of capped RNA and its interactions with proteins and antibodies.
Cap-Binding Proteins and Antibody Targeting
You find that cap-binding proteins work together in complexes to recognize and bind capped mRNA. The nuclear cap-binding complex (CBC) contains proteins like NCBP1, NCBP2, and NCBP3. These proteins interact with each other and may also serve as targets for antibodies. Co-immunoprecipitation experiments show that NCBP2 binds to NCBP3, forming dynamic interactions. The presence of all three proteins in the same ribonucleoprotein particles suggests a structural relationship that helps antibodies find their targets.
| Protein | Role in CBC | Interaction Evidence |
|---|---|---|
| NCBP1 | Core CBC component | Found in RNPs with NCBP2/3 |
| NCBP2 | Binds NCBP3 | Co-IP experiments |
| NCBP3 | Dynamic partner | Structural studies |
You can use antibodies to study these complexes and learn how the binding cap affects the recognition of capped mRNA. This knowledge helps you design better experiments and develop new antibody therapies.
Methods for Binding Cap Analysis

Understanding how to analyze the binding cap is key for antibody development. You can use several methods to study how antibodies interact with their targets. These methods help you see the structure, measure the strength of binding, and predict how changes affect function. You can apply these tools to both protein and mRNA binding caps.
Structural Techniques
You can use structural techniques to see how the binding cap fits with its target. Two main methods are X-ray crystallography and cryo-electron microscopy (cryo-EM).
- X-ray crystallography gives you atomic-level details. You can use it for smaller proteins or complexes under 100 kDa. This method helps you see exactly where each atom sits in the binding cap.
- Cryo-EM works well for large, multi-component structures. You can see different shapes and states that the binding cap might take. This method captures dynamic interactions that X-ray crystallography may miss.
- Different conditions for cryo-EM and crystallization can show you various biological states. You can use the crystal structure as a model to fit into the shapes you see with cryo-EM. Cryo-EM can also help you design better crystals for X-ray studies.
These techniques let you compare how the binding cap interacts with proteins or mRNA. You can use them to guide your research and improve antibody design.
Biochemical Assays
You need biochemical assays to measure how well the binding cap works. These tests help you with detection, quantitative measurement, and characterization of antibody interactions. You can use them to test both protein and mRNA targets.
| Assay Type | Advantages | Limitations |
|---|---|---|
| Cell-Binding Assays | Speed and ease of execution | Limited in measuring high-affinity interactions |
| Kinetic Exclusion Assay | Direct measurement of free receptor at equilibrium | More complex setup required |
| Surface Plasmon Resonance | Measures binding kinetics | Requires specialized equipment |
You can also use Surface Plasmon Resonance (SPR) and Biolayer Interferometry. These methods help you measure binding kinetics and see how fast and strong the binding cap attaches to its target.
When you choose an assay, you should think about detection sensitivity and specificity. Some assays work better for screening, while others give you more quantitative results.
| Assay Type | Analytic Sensitivity | Analytic Specificity | Clinical Sensitivity | Clinical Specificity |
|---|---|---|---|---|
| Screening Assays | 91.2% | 99.6% | 82.4% | 89.0% |
| Fractionation Assays | 87.2% | 93.9% | N/A | N/A |
| MPS-I/II | 96.5% | N/A | N/A | N/A |
| MPS-III | 88.1% | N/A | 88.2% | N/A |
| MPS-IV | 78.8% | N/A | N/A | N/A |
| MPS-VII | N/A | N/A | 79.3% | N/A |
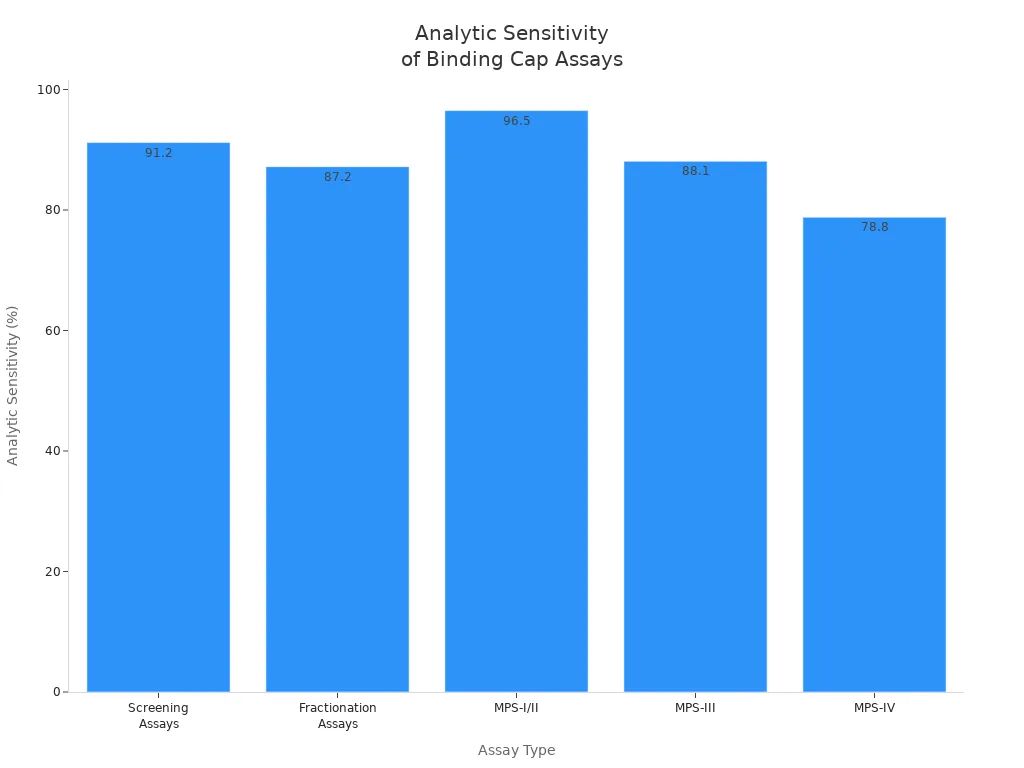
You can see that screening assays have high analytic sensitivity and specificity. This helps you pick the best affinity reagents and monoclonal antibodies for your research.
Computational Tools
You can use computational tools to model and predict how the binding cap interacts with its target. These tools help you with detection and quantitative measurement before you do experiments in the lab.
- ZDOCK lets you predict how two proteins or a protein and mRNA will fit together.
- UCSF Chimera and PyMOL help you visualize and analyze the 3D structure of the binding cap.
- DLAB, CSM-AB, DSMBind, TAP, and PropertyDAG help you predict binding strength and find important contact points.
You can use these tools to screen many antibody candidates quickly. They help you focus on the best options for further testing. Recent studies show that binding energy calculations can identify up to 89% of important mutations in protein complexes. This means you can trust these tools for accurate characterization and quantitative measurement.
You can combine structural, biochemical, and computational methods for a complete view of the binding cap. This approach helps you improve detection sensitivity, optimize binding, and speed up antibody development.
Analyzing Binding Kinetics
Measuring Antibody Interactions
You need to measure how antibodies interact with their targets to understand binding kinetics. This process helps you see how quickly and strongly an antibody attaches to a binding cap. You can follow a standard protocol to get reliable results:
- Select the right chip and microplate volume for your experiment.
- Place activators, ligands, and analytes in the correct wells.
- Immobilize the ligand by injecting it at set flow rates and contact times.
- Regenerate the surface using glycine and PBS-T-EDTA.
- Inject antibodies and analytes, making sure to control association and dissociation times.
- Use ProteOn Manager software to analyze the data and get kinetic parameters.
You can use different tools to measure antigen-antibody interactions. Surface Plasmon Resonance (SPR) and Biolayer Interferometry (BLI) are two common methods. Both give you real-time data and do not need labels. SPR uses a gold-coated chip to hold one binding partner. You inject the other partner and watch the interaction as it happens. BLI uses optical tips that dip into wells with analytes. You can compare these two methods using the table below:
| Feature | Biolayer Interferometry (BLI) | Surface Plasmon Resonance (SPR) |
|---|---|---|
| Pros | High throughput, Low vibrational/mechanical noise | Very reproducible, Low-cost, Minimal sample consumption |
| Cons | Poor reproducibility, Relatively high sample consumption, Should be cross-validated with SPR | Low throughput |
Tip: Both SPR and BLI give you sensorgrams, which are graphs that show the binding events over time. SPR measures changes in refractive index, while BLI measures changes in optical depth.
You can use these methods to get a clear picture of how antibodies bind to their targets. This helps you with detection and characterization of binding kinetics in your research.
Data Interpretation
You need to look at several key parameters when you interpret binding kinetics data. These values help you understand how well the antibody binds and how stable the interaction is. The table below shows the main parameters you should check:
| Parameter | Description |
|---|---|
| Association rate constant (kon) | Shows how fast the antibody binds to the cap. |
| Dissociation rate constant (koff) | Tells you how quickly the antibody-cap complex falls apart. |
| Equilibrium dissociation constant (KD) | KD = koff/kon. This value tells you the affinity of the antibody for the cap. |
| Maximum response (Rmax) | The highest response level you see during the binding event. |
You can use these numbers to compare different antibodies. A low KD means the antibody has high affinity for the cap. A high kon shows the antibody binds quickly. A low koff means the complex stays together longer. Rmax tells you how much binding happens at the peak.
Note: Always check your sensorgrams for unusual shapes or noise. These can show problems with the assay or the sample.
You can use these results to guide your next steps in antibody development. Careful analysis of binding kinetics helps you pick the best candidates for further testing and improves your understanding of antigen-antibody interactions.
Step-by-Step Workflow
Sample Preparation
You start your binding cap analysis by preparing your samples carefully. First, you need to optimize the cell concentration. This step helps you get clear results. You should block non-specific binding to lower background signals. Add your antibody cocktails to the cell samples. Incubate them in the dark at 4°C for 20 to 30 minutes. This temperature keeps the antibodies from being taken up by the cells and ensures strong binding. After incubation, wash the samples well to remove any unbound antibodies. If you cannot analyze the samples right away, use a fixation solution to keep them stable. Good sample preparation gives you reliable data and helps you avoid errors later.
Data Collection
You collect data using methods that give you the most accurate results.
- Measure the Dynamic Binding Capacity (DBC) by loading a column with a known amount of sample. Set the flow rate and buffer conditions to help the binding process.
- Use a sample that represents your real material, such as Harvest Cell Culture Fluid (HCCF), when you test Protein A affinity resin for monoclonal antibodies.
- Fractionate the flow-through to check for breakthrough material. This step tells you if the column has reached its maximum DBC. Always consider the precision and resolution of your assay during this process.
These steps help you gather high-quality data for your research.
Troubleshooting
You may face problems during your experiments. Use these tips to solve common issues:
- Monitor environmental factors like temperature, humidity, and light to keep your results consistent.
- Address baseline drift by using the right regeneration buffers and checking if your buffers are compatible.
- Optimize interaction kinetics by adjusting flow rates or changing experimental conditions to improve binding and dissociation.
- Minimize non-specific binding by using surface blocking agents and improving surface chemistry.
Tip: Careful troubleshooting helps you get clear and reproducible results every time.
Applying Results to Antibody Development
Candidate Optimization
You can use binding cap analysis to make your antibody candidates better. This process helps you improve antibody specificity, affinity, and even mRNA targeting. Many researchers use structure-based computational design to change the binding cap. For example, you might see a 4.6-fold increase in affinity for an antibody like 11K2 after such changes. In silico affinity maturation can boost antigen binding by more than 100 times in some cases.
You can try these strategies to optimize your candidates:
- Use deep mutagenesis to explore more antibody sequences. This gives you a better chance to find strong drug leads.
- Apply machine learning models to predict which sequences will improve binding. This saves time during screening.
- Use affinity maturation methods, such as random or targeted mutagenesis, to make antibodies bind better to their targets.
- Optimize several properties at once. This helps you avoid problems with binding affinity or developability.
- Add natural antibody sequences to your models. This step can improve predictions for immunogenicity and developability.
These steps help you select the best candidates for further research and detection.
Case Examples
You can see real improvements in antibody development when you use binding cap analysis. The Antibody Random Forest Classifier (AbRFC) helped scientists create antibodies with over 1000-fold higher affinity against Omicron subvariants like BA.1, BA.2, and BA.4/5. The second-round candidate, CMAB262, showed more than a 1000-fold increase in affinity against BA.1 by combining the best mutations from earlier rounds. Another candidate, GMAB156, improved its affinity by 317 times compared to its earlier version. These examples show that you can keep important properties across different strains while also gaining new benefits from specific mutations.
You can use binding kinetics data and detection methods to guide your choices. These case studies show how careful analysis leads to better antibodies for both research and therapy.
You can analyze binding cap interactions by following clear steps. Use structural, biochemical, and computational methods to get a full picture. Measure how antibodies bind and check the results for accuracy. When you combine these tools, you improve antibody specificity and affinity. For deeper knowledge, explore the article “Structure of apo-CAP reveals that large conformational changes are necessary for DNA binding.” This research can help you advance your antibody development skills.
FAQ
What is a binding cap in antibody development?
A binding cap is a special region on an antibody. You use it to help the antibody attach to its target, like a protein or mRNA. This cap controls how well the antibody recognizes and binds to its target.
Why do you analyze binding cap interactions?
You analyze binding cap interactions to improve antibody specificity and strength. This helps you design better antibodies for research, therapy, or diagnostics. Strong binding means your antibody works more effectively.
Which methods can you use to study binding cap interactions?
You can use structural techniques (like X-ray crystallography), biochemical assays (such as ELISA), and computational tools (like molecular modeling). Each method gives you different information about how the binding cap works.
How do binding caps affect mRNA recognition?
Binding caps help antibodies and proteins tell the difference between capped and uncapped mRNA. This selectivity lets you target only the right mRNA molecules, which is important for gene regulation and therapy.
Can you improve antibody performance by changing the binding cap?
Yes! You can use mutagenesis or computational design to change the binding cap. These changes often increase binding strength or specificity. For example, some studies show a 4.6-fold increase in affinity after optimizing the binding cap.

