News & Events
Apolipoprotein A-1 or MBP as Fusion Partners for Antibody Production, What Does the Research Say
Current research shows that MBP often leads to higher solubility and easier purification in antibody production when compared with apolipoprotein a-1. Scientists use four main criteria to compare these fusion partners: solubility, yield, purification, and immunogenicity. Choosing the right fusion partner can improve the success of antibody production and save time in the lab.
Key Takeaways
- Choosing the right fusion partner, like MBP or Apolipoprotein A-1, can significantly enhance antibody production by improving solubility and yield.
- MBP often leads to higher solubility and yield compared to Apolipoprotein A-1, making it a preferred choice in many experiments.
- Understanding purification methods is crucial; MBP fusion proteins can be easily purified using nickel affinity chromatography, achieving high recovery rates.
- Researchers should match fusion partners to their specific experimental needs and host systems to optimize results and reduce costs.
- Staying informed about emerging fusion partners and advances in antibody production technology can lead to better outcomes in research and therapy.
Evaluation Criteria
Understanding the main criteria for evaluating fusion partners helps researchers choose the best option for antibody production. The table below shows the most commonly cited criteria in scientific literature:
| Criteria | Description |
|---|---|
| Developability profile | Properties that show if a candidate can become a safe and effective drug. |
| Production yield | The amount of antibody made during the process. |
| Biophysical stability | The ability of the antibody to keep its shape and function in different conditions. |
| Solubility | How well the antibody dissolves in a solution, which affects its use in experiments. |
| Polyreactivity | The chance that the antibody binds to more than one target, which can lower its specificity. |
| Immunogenicity | The likelihood that the antibody will cause an immune response in the host. |
Solubility
Solubility measures how easily a protein or antibody dissolves in a liquid. High solubility means the protein stays in solution and does not form clumps. This property is important because it helps researchers get more usable antibody from each experiment. Proteins with low solubility can be hard to work with and may lower the success rate of antibody production.
Yield
Yield refers to the total amount of antibody produced during the process. A higher yield means more product is available for research or therapy. Scientists look for fusion partners, such as apolipoprotein a-1, that can increase yield and make the process more efficient.
Immunogenicity
Immunogenicity is the ability of a protein to trigger an immune response. Researchers want to control immunogenicity to avoid unwanted reactions. Studies show that fusing peptides with certain proteins can boost antibody production by making the immune system respond more strongly. For example:
- Fusion with expression enhancers can increase antibody yield.
- Some fusion partners, like StxB, can help the immune system recognize and respond to the target protein.
Purification
Purification is the process of separating the desired antibody from other substances. Efficient purification methods help researchers get a pure product quickly. Common methods include cation exchange chromatography, which removes unwanted molecules, and hydrophobic interaction chromatography, which helps polish the final product. These methods often achieve over 90% yield, making them reliable for antibody production.
Apolipoprotein A-1 as Fusion Partner
Role in Protein Expression
Apolipoprotein a-1 plays a key role in protein expression systems. Researchers use it to improve the production of recombinant proteins. Studies show that expression yields can reach up to 80 μg/ml, which was the highest reported at the time. However, scientists observed some degradation of apolipoprotein a-1. When they used protease inhibitors like pepstatin and chymostatin, the yield increased, and degradation decreased. The table below summarizes these findings:
| Key Findings | Details |
|---|---|
| Expression Yield | Up to 80 μg/ml achieved; optimal conditions yield 20-30 μg/ml without degradation |
| Degradation | Immunoblot analysis showed degraded apolipoprotein a-1 |
| Fragments | Fragments of 26 and 14 kDa observed, matching in vivo results |
| Protease Inhibitors | Increased yield and reduced degradation |
Solubility and Yield
Scientists value apolipoprotein a-1 for its ability to increase solubility in fusion proteins. This property helps keep proteins in solution and prevents clumping. Higher solubility leads to better yields, making experiments more successful. Researchers found that using protease inhibitors can further boost yield by limiting protein breakdown. These results suggest that apolipoprotein a-1 can help produce more usable antibody in each experiment.
Immunogenicity
Apolipoprotein a-1 fusion proteins show interesting effects on the immune system. Animal studies reveal several points:
- ApoA-I mimetics showed promise in preclinical models, but recent clinical trials for heart disease did not show clear benefits.
- Future studies may reveal more about the immunogenicity and therapeutic potential of apolipoprotein a-1 mimetics.
Researchers also found that fusing interferon alpha with apolipoprotein a-1 reduced harmful effects in mice. The fusion led to strong antitumor responses, including tumor removal in many treated mice. Scientists observed more tumor-specific T cells and increased granzyme B in NK cells, showing a clear immune response.
Purification Methods
Researchers use different methods to purify apolipoprotein a-1 fusion proteins. The most effective protocols depend on sample size and desired purity. The table below compares two common methods:
| Purification Method | Yield | Purity | Notes |
|---|---|---|---|
| Gel-based method | Higher than chromatography | High | Best for small samples (1-2 ml); faster isolation |
| Chromatography | Lower per sample | High | Better for large-scale purifications; less effective for small samples |
Gel-based methods work well for small volumes and provide quick results. Chromatography suits larger batches but may not be as efficient for small samples. Both methods produce pure apolipoprotein a-1 with properties similar to the native protein.
MBP as Fusion Partner
Role in Protein Expression
Researchers often choose MBP, or maltose-binding protein, as a fusion partner to improve protein expression. Studies show that MBP helps both bacterial and eukaryotic cells produce more protein. When scientists fuse hydrophobic membrane proteins with MBP, they see better stability and higher expression levels. MBP acts as a solubility enhancer, helping proteins fold correctly and making them easier to study. In mammalian cells, MBP boosts the expression of complex proteins, including those with disordered regions. This versatility makes MBP a popular choice for many types of experiments.
Solubility and Yield
MBP fusion proteins show strong solubility and yield outcomes. The position of MBP in the fusion construct affects these results. The table below summarizes findings from recent studies:
| Positioning of MBP | Solubility Outcome | Yield Outcome |
|---|---|---|
| N-terminal | Superior | Higher |
| C-terminal | Inferior | Lower |
| Minor linker changes | Significant impact | Significant impact |
Placing MBP at the N-terminal end leads to better solubility and higher yields. Small changes in linker residues can also make a big difference. Researchers working with large eukaryotic proteins or those with complex structures often prefer MBP for these reasons. MBP’s performance compares favorably to other fusion partners, such as apolipoprotein a-1, especially in bacterial systems.
Immunogenicity
Scientists have studied the immunogenicity of MBP fusion proteins in animal models. Serological tests using cattle with Johne’s disease show that MBP fusion proteins can trigger antibody responses. Some animals produce antibodies against all tested MBP fusion proteins, while others show a weaker response. This variability means MBP fusion proteins have immunogenic potential, but the response may differ between individuals.
Purification Methods
MBP fusion proteins are easy to purify using affinity chromatography. Researchers report high recovery rates with nickel resin methods. The table below shows the efficiency of different purification techniques:
| Purification Method | Monomer Recovery Rate (%) | Notes |
|---|---|---|
| Nickel Resin N1 | 95 | Highest monomer recovery for MBP-16E6mut |
| Nickel Resin N2 | 87 | Effective for both MBP-16E6mut and MBP-8E6 |
| Nickel Resin N4 | 85 | High recovery capacity of total purified protein |
| Amylose Resin | 16 | Retains higher proportion of oligomers for MBP-16E6mut |
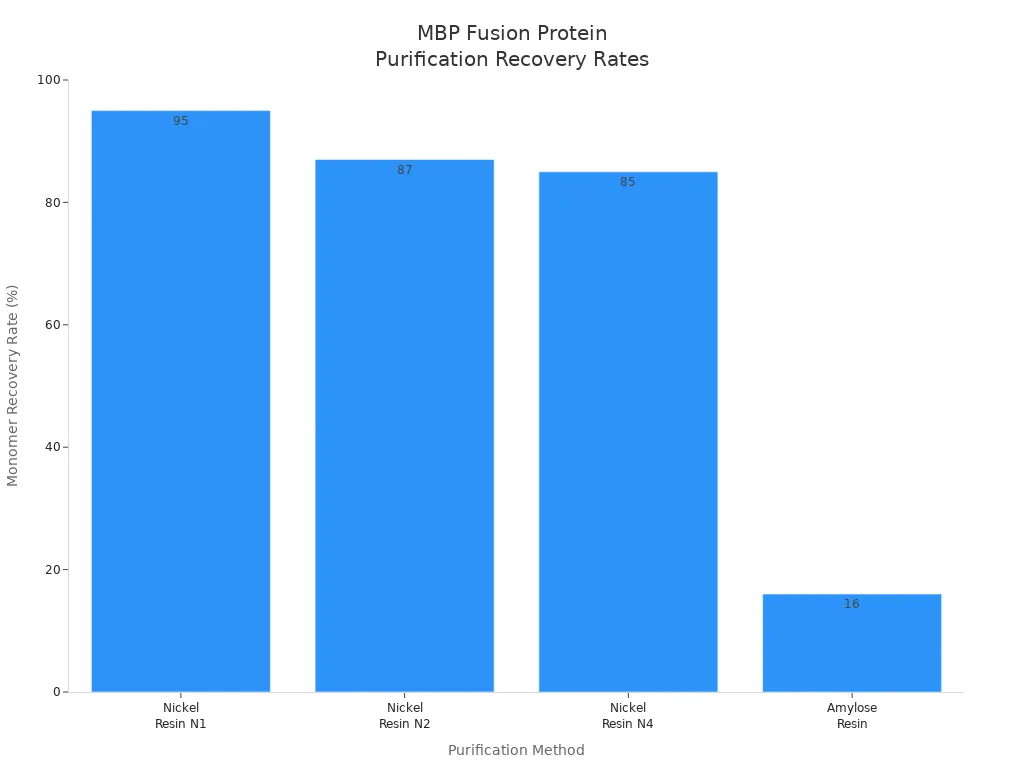
Nickel resin methods provide the highest monomer recovery rates, making them the preferred choice for MBP fusion protein purification.
Apolipoprotein A-1 vs MBP
Solubility Comparison
Solubility plays a key role in antibody production. Researchers have compared apolipoprotein a-1 and MBP in several studies. MBP often increases the solubility of fusion proteins more than apolipoprotein a-1. Scientists found that MBP, especially when placed at the N-terminal end, keeps proteins in solution and prevents clumping. This property helps researchers recover more functional protein. Apolipoprotein a-1 also improves solubility, but its effect is usually less pronounced than MBP. In bacterial systems, MBP stands out as the preferred solubility enhancer.
Note: High solubility reduces the risk of protein aggregation and loss during purification.
Yield Comparison
Yield measures how much antibody or protein is produced. MBP fusion constructs often deliver higher yields than those with apolipoprotein a-1. Studies show that MBP can boost protein expression in both bacterial and eukaryotic cells. For example, MBP fusions can reach yields that surpass 100 μg/ml in optimized systems. Apolipoprotein a-1 can achieve up to 80 μg/ml under ideal conditions, but yields often drop without protease inhibitors. MBP’s ability to stabilize proteins during expression leads to more consistent and higher yields.
| Fusion Partner | Typical Yield (μg/ml) | Notes |
|---|---|---|
| MBP | 100+ | High yield in both bacteria and eukaryotes |
| Apolipoprotein a-1 | Up to 80 | Yield drops without protease inhibitors |
Immunogenicity Comparison
Immunogenicity affects how the immune system responds to the fusion protein. MBP fusion proteins can trigger antibody responses in animal models, but the strength of the response varies. Some animals produce strong antibodies against MBP, while others show weaker reactions. Apolipoprotein a-1 fusions also influence immune responses. In some studies, fusing therapeutic proteins with apolipoprotein a-1 reduced harmful immune effects and increased tumor-specific immune activity in mice. However, clinical trials using apolipoprotein a-1 mimetics for heart disease did not show clear benefits. Both fusion partners can affect immunogenicity, but their effects depend on the target protein and the host system.
- MBP: Variable antibody response; strong in some animals.
- Apolipoprotein a-1: Can enhance immune response to fused proteins; clinical results mixed.
Purification Comparison
Purification methods determine how easily researchers can isolate the desired protein. MBP fusion proteins are easy to purify using affinity chromatography, especially with nickel resin. Recovery rates for MBP fusions can reach up to 95%. This method works well for both small and large sample volumes. Apolipoprotein a-1 fusion proteins can be purified using gel-based methods or chromatography. Gel-based methods work best for small samples and provide high purity, while chromatography is better for large-scale production but may yield less per sample.
| Fusion Partner | Preferred Purification Method | Recovery Rate (%) | Best For |
|---|---|---|---|
| MBP | Nickel affinity chromatography | Up to 95 | All sample sizes |
| Apolipoprotein a-1 | Gel-based or chromatography | High (small scale) | Small or large samples |
Researchers often choose MBP for its simple and efficient purification process, especially when working with large batches.
Practical Considerations
Experimental Contexts
Researchers select fusion partners based on the needs of their experiments. The type of antibody required often depends on the target protein’s abundance and the experiment’s purpose. Some antibodies work better for detecting rare proteins, while others suit more common targets. Scientists also consider the specificity of the antibody. The structure of both the antibody and the antigen, including how the epitope folds, can affect binding. The production and purification process matters as well. If the process lacks control, the antibody may lose specificity. Experimental conditions, such as the concentration of the antibody and the environment in which it is used, also play a role in how well the antibody binds to its target.
Tip: Always match the fusion partner to the experiment’s requirements for best results.
- Type of experiment and target protein abundance
- Antibody specificity and epitope folding
- Production and purification control
- Optimal antibody concentration and experimental conditions
Host Systems
The choice of host system can change how well apolipoprotein a-1 or MBP works as a fusion partner. E. coli is a popular host because it grows quickly and costs less. It works well for small proteins and simple fusion constructs. Mammalian cells handle larger proteins and add important modifications, but they require more resources and time. Yeast systems, like P. pastoris, offer a balance between the two. They can produce proteins with some modifications and higher yields than bacteria. The complexity of the host system often matches the complexity needed for the protein’s function.
Scalability
Scalability means how easily a process can move from small experiments to large production. MBP fusion proteins scale well in bacterial systems like E. coli. Researchers can produce large amounts at low cost. Apolipoprotein a-1 fusions may need more careful handling, especially in larger systems. Mammalian and yeast hosts can scale up, but they often require more steps and resources.
Cost and Resources
Cost and resource needs differ between fusion partners and host systems. E. coli systems with MBP are usually the most affordable and need fewer resources. Mammalian cell systems cost more and take longer but may be necessary for complex proteins. Yeast systems fall in the middle. Researchers should weigh the benefits of higher yield and better modifications against the extra time and money required.
Choosing the right fusion partner and host system helps save time, reduce costs, and improve antibody quality.
Future Trends
Emerging Fusion Partners
Researchers continue to search for new fusion partners that can improve antibody production. SIPH and SILC cell lines have gained attention in recent studies. Scientists have tested several SIPH cell lines, such as SIPH1, SIPH5, SIPH7, and SIPH16, for their ability to produce immunogen-specific monoclonal antibodies. These cell lines have shown promise by generating stable clones that keep producing antibodies over time. SILC4-Ag1 cells also work as fusion partners, making suncus monoclonal antibodies, although these are not always specific to the immunogen.
- SIPH cell lines help create stable antibody-producing cells.
- SILC4-Ag1 cells produce suncus antibodies, but not always immunogen-specific.
- ELISA tests confirm that some SIPH cell fusions make immunogen-specific antibodies.
- SIPH7A2G25 cells show potential, with several fusion cells staying stable and making antibodies.
Scientists expect that new fusion partners like SIPH and SILC will help create better antibodies for research and therapy.
Advances in Antibody Production
Recent technology has changed how researchers make antibodies. Karyochi-based human hybridomas now produce full-size immunoglobulins with both antigen-binding and effector domains. This method solves problems found in older techniques and gives stable, high-yield production of fully human monoclonal antibodies. Hybridoma technology lets scientists make antibodies against almost any antigen. Electroporation and electrofusion methods improve fusion efficiency, giving high yields and low cell damage.
- Karyochi-based human hybridomas produce stable, fully human antibodies.
- Hybridoma technology supports antibody production for many antigens.
- Electroporation and electrofusion increase fusion yield and reduce cell harm.
- Human Karyochi cells can fuse with antibody-secreting lymphocytes, removing the need for expensive humanization steps.
These advances make antibody production faster, more efficient, and more cost-effective. Researchers can now create high-quality antibodies for many uses in medicine and science.
Researchers often prefer MBP as a fusion partner for antibody production because it improves solubility, yield, and purification. Apolipoprotein a-1 also helps but works best in specific cases. Scientists should match fusion partners to their experiment needs and host systems. Future studies may focus on:
- Using chaperone proteins for stability
- Designing polymerizing tags with synthetic biology
- Exploring glycosylation and sialylation effects
- Accelerating clinical use of Fc-fusions
Staying updated with new technologies helps researchers achieve better results.
FAQ
What is a fusion partner in antibody production?
A fusion partner is a protein scientists attach to another protein to help with expression, solubility, or purification. This method makes it easier to produce and study antibodies in the lab.
Why do researchers choose MBP over other fusion partners?
Researchers often select MBP because it increases solubility and yield. MBP also allows for simple purification using affinity chromatography. These benefits make MBP a popular choice in many experiments.
Can apolipoprotein a-1 improve antibody production?
Apolipoprotein a-1 can help increase solubility and yield in some cases. Scientists have used it to boost protein expression, especially when protease inhibitors are present to prevent degradation.
What host systems work best for fusion protein production?
E. coli works well for small proteins and MBP fusions. Mammalian cells handle larger proteins and complex modifications. Yeast systems offer a balance between yield and complexity.
Are MBP fusion proteins safe for therapeutic use?
MBP fusion proteins can trigger immune responses in animals. Researchers must test each fusion protein for safety before using it in therapy or medicine.