Antibody knowledge
Lab 34 Peptides and Proteins: Surprising Facts You Never Knew
Did you know that a single drop of acid can cause a protein to lose its shape in seconds?
When you explore lab 34 peptides and proteins, you discover how tiny changes can transform the structure of a protein.
In lab 34 peptides and proteins, you test how a protein reacts to heat, salt, or even light.
You might see a protein unravel and become useless just by changing the temperature in lab 34 peptides and proteins.
Many students in lab 34 peptides and proteins find that a protein can surprise you, acting differently than you expect.
You will challenge your ideas about protein every time you work in lab 34 peptides and proteins.
Key Takeaways
A single change in temperature or pH can cause proteins to lose their shape. Understanding this helps you predict how proteins behave in different environments.
Peptides and proteins differ mainly in size. Peptides have fewer than 50 amino acids, while proteins can have hundreds or thousands. This affects their structure and function.
Misfolded proteins can lead to serious diseases. Learning about protein folding can help you understand health issues like Alzheimer’s and Parkinson’s.
Rare amino acids play a crucial role in protein function. They can enhance stability and give proteins unique abilities, making them important in medicine.
Peptides can be used in targeted drug delivery systems. They help deliver medications directly to diseased cells, improving treatment effectiveness.
Lab 34 Peptides and Proteins: Structure
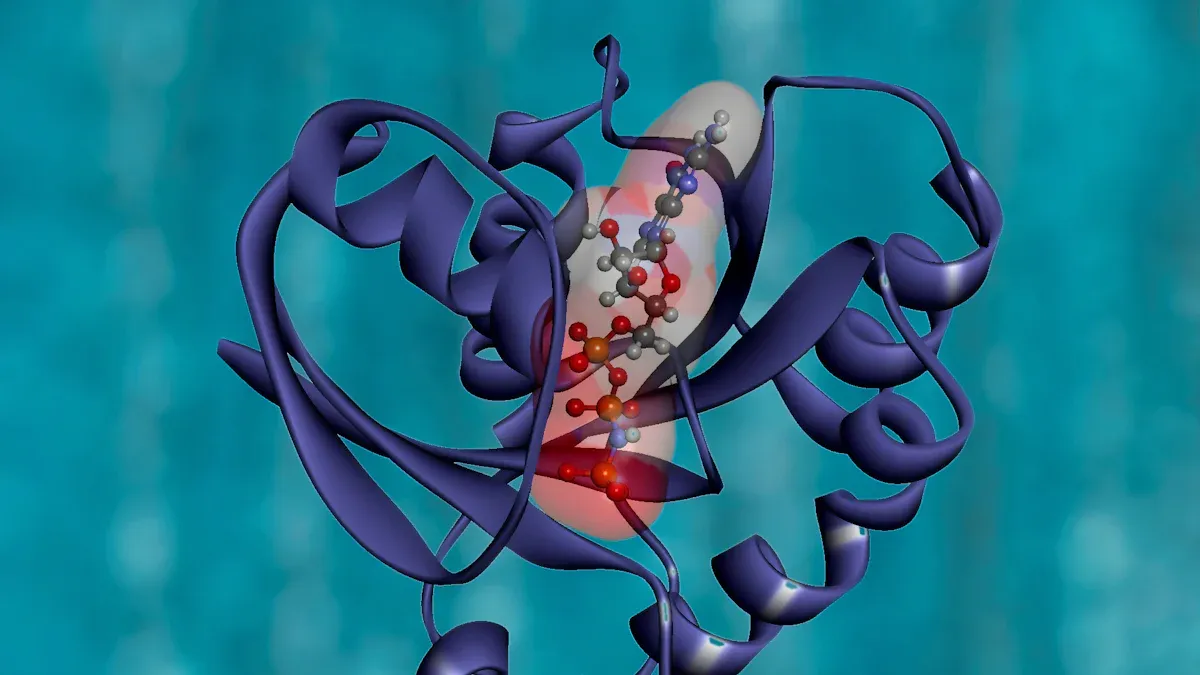
Peptide Bonds
You can spot the difference between peptides and proteins by looking at their chain length. Peptides usually have fewer than 50 amino acids, while proteins can have hundreds or thousands. The peptide bond forms when the amino group of one amino acid joins the carboxyl group of another. This bond is not like other chemical bonds in biological molecules. Peptide bonds show double bond character, which makes them rigid and planar. You will notice that the flexibility of a polypeptide chain comes from rotations around the alpha-carbon atoms, not the peptide bond itself. This rigidity helps maintain protein structure. Some peptide bonds, especially those with aspartic acid, break down more easily. This can affect the stability of protein therapeutics. In extremophiles, proteins show more non-planar peptide bonds, which increases their compactness and stability. You can see how peptide bonds play a key role in protein structure and function.
Protein Folding
Protein folding is a process that gives each protein its unique shape. You will see that proteins fold into complex patterns, such as alpha-helices and beta-pleated sheets. Sometimes, proteins misfold and form entanglements. These misfolded states can last a long time and change the way proteins behave. Laboratory studies show that removing misfolding events leads to normal folding patterns. Misfolded proteins can match structural signals seen in refolded proteins. This means that protein structure can change in unexpected ways. X-ray crystallography helps you study these folding patterns and understand protein structure. You can use structural determination to see how folding affects protein function.
Rare Amino Acids
Rare amino acids add diversity to protein structure and function. You will find that cell-penetrating peptides and antimicrobial peptides use special amino acids to change their secondary structure and function. These rare amino acids can make proteins more stable or give them new abilities. For example, selenocysteine protects against oxidative stress, and hydroxyproline helps collagen stay strong. The side chains of rare amino acids influence protein structure by changing local conformations. You can use techniques like high-performance liquid chromatography and x-ray crystallography to identify rare amino acids in natural polypeptides. These methods help you with structural determination and reveal how rare amino acids affect protein structure and function.
Technique | Description | Sample Size |
|---|---|---|
High-performance liquid chromatography | Used for micro-level analysis of unusual amino acids | ∼10 pmol |
High-performance capillary electrophoresis | Effective for identifying rare amino acids | ∼1–10 pmol |
Matrix-assisted laser desorption ionization time-of-flight mass spectrometry | Identifies modified amino acids | ∼1–10 pmol |
Automatic protein gas phase sequencing | Useful for sequencing peptides | ∼1–50 pmol |
Tip: You can use x-ray crystallography to see the exact arrangement of atoms in a protein structure. This helps you understand how rare amino acids change protein structure and function.
Properties of Peptides and Proteins
Denaturation Factors
You can change the properties of a protein by exposing it to different conditions. In the lab, you often see proteins lose their shape and stop working when you heat them or add chemicals. This process is called denaturation. When a protein denatures, it unfolds and loses its special structure. You can see this when you cook an egg. The clear egg white turns solid because the heat distorts the proteins and causes them to stick together.
Denaturation disrupts the bonds that hold the protein’s secondary, tertiary, and quaternary structures. The protein chain unravels, and the protein loses its original properties and function.
Here are some common factors that cause protein denaturation in laboratory experiments:
Heat
Radiation
Repeated freezing
High pressure
Acids and alkalis
Organic solvents
Concentrated urea solutions
You can also use chemicals like urea, guanidinium chloride, oxidizing agents, and reducing agents to break down protein structure. Sometimes, denaturation is reversible if the disruption is mild. For example, if you gently heat a protein, it might refold and regain its function when cooled.
Denaturation Factor | Description |
|---|---|
Physical Agents | Heat, radiation, repeated freezing, and high pressure can alter protein conformation. |
Chemical Agents | Acids, alkalis, organic solvents, and concentrated urea solutions disrupt protein structure. |
Environmental Conditions | Extremes of pH or temperature can lead to loss of protein solubility and biological activity. |
You can measure how temperature affects protein stability. For example, the binding of a phosphorylated peptide to a protein at 37°C shows a strong stabilization effect, with a binding constant of 2.3 × 10^5 M−1. Adding 1 mM of this peptide can stabilize the protein by about 10°C. On the other hand, adding 1 mM of another molecule, ANS, can destabilize the protein by about 10°C. This shows how small changes in the environment can make a big difference in protein stability.
Dual Functions
Some proteins surprise you with their ability to perform more than one job in the cell. These dual-function proteins help cells adapt to changing conditions. For example, KLHL proteins act as adaptors for Cullin 3-RING ligases. They help tag other proteins for destruction, which keeps the cell clean and organized. At the same time, they play a role in organizing the cell’s skeleton and responding to stress from harmful molecules.
When you study these proteins, you see how their dual roles support cell health. If these proteins stop working, cells can develop problems, and diseases may occur. You can appreciate how a single protein can have more than one function, making it essential for survival and adaptability.
Environmental Response
Proteins must adapt to changes in their environment to keep working. You can see this when you change the pH or add chemicals in the lab. Proteins use several tricks to stay stable and keep their function.
Adaptation Aspect | Description |
|---|---|
Charges help stabilize protein interactions and complexes, especially when pH changes. | |
Ionizable Groups | These groups can change their charge, which affects protein stability and function. |
Ionic Milieu Influence | The surrounding ions, like calcium, can change how proteins behave and interact. |
Factor | Influence |
|---|---|
Evolutionary History | Proteins adapt over time, changing their charge distribution to survive in different pH levels. |
Mutational Bias | Mutations can change how proteins work in new environments. |
Gene Expression | Cells can make more or less of a protein to help it adapt to new conditions. |
You can use different experiments to study how proteins respond to the environment:
Laboratory experiments let you control variables like temperature and pH.
Field experiments show how proteins work in real-world settings.
Gene and protein expression analyses help you see how cells react at the molecular level.
Global gene expression profiling reveals how cells adjust to stress.
qPCR-based analysis compares how single genes respond to changes.
Quantitative proteomics measures changes in protein levels and modifications.
Tip: When you test proteins in the lab, always remember that their environment can change their structure and function. This adaptability helps proteins survive in many different conditions.
Peptide Synthesis and Techniques
Solid-Phase Peptide Synthesis
You can create peptides in the lab using solid-phase peptide synthesis. This method lets you build a peptide chain one amino acid at a time. You start by anchoring the first amino acid to a resin bead. This keeps the growing chain attached and easy to handle. You then remove the protecting group from the amino acid and add the next one. You repeat these steps until you finish the sequence. When the chain is complete, you use a strong acid to cut the peptide from the resin and remove any side-chain protecting groups. You then purify the peptide and check its quality.
Here are the main steps you follow in solid-phase peptide synthesis:
Anchor the first amino acid to the resin.
Remove the protecting group.
Add the next amino acid.
Repeat the deprotection and coupling steps.
Cleave the peptide from the resin.
Purify and check the peptide.
You can choose from different types of polymer carriers and linkers. Each type has special features that help with peptide chain growth and stability. The table below shows some common options:
Type of Polymer Carrier | Description |
|---|---|
Polystyrene-phenylene diethylene crosslinked resin | Must contain suitable connecting molecules and be stable during synthesis. |
Polyacrylamide | Provides sufficient junctions for peptide chain growth. |
Polyethylene-ethylene glycol resin | Must be stable and not react with amino acids. |
Chloromethyl resin | A type of resin with specific connecting molecules. |
Carboxyl resin | Another type of resin used in SPPS. |
Amino resin | Used for specific peptide synthesis needs. |
Hydrazide resin | A resin type that can be used based on peptide requirements. |
You also use different linkers to connect the first amino acid to the resin. Some linkers, like chloromethyl and succinyl, are very common. Others, like o-nitrobenzyl, allow for special cleavage methods.
Linker Type | Characteristics |
|---|---|
Bifunctional compounds | Must be stable and allow for quantitative cleavage post-synthesis. |
Chloromethyl | Commonly used linker type. |
Mercapto | Another linker option. |
Acyl chloride | Used for specific peptide structures. |
p-benzoyl | A linker type with specific applications. |
Aryl sulfonyl chloride | Used in various peptide synthesis processes. |
Allyl | A versatile linker type. |
Succinyl | Commonly used in SPPS. |
o-nitrobenzyl | A linker that allows for specific cleavage. |
Diphenylchlorosilane | Another linker option. |
You form peptide bonds using several methods. The condensation agent method is very common. The mixed anhydride method helps reduce side reactions. The activated ester method is popular because it prevents racemization. The DCC and HOBT methods are also widely used for their effectiveness.
Peptide Bond Formation Method | Description |
|---|---|
Condensation agent method | Commonly used for peptide bond formation. |
Mixed anhydride method | Reduces side reactions during synthesis. |
Acyl chloride method | Effective in peptide bond formation. |
Activated ester method | Widely used to inhibit racemization. |
DCC method | A popular method for peptide bond formation. |
HOBT method | Reduces side reactions effectively. |
Tip: You can use high-performance liquid chromatography and mass spectrometry to check the purity and identity of your peptide after synthesis.
Automated Methods
You can now use automation to make peptide synthesis faster and more accurate. Automated machines handle each step, from adding amino acids to washing and deprotection. This reduces mistakes and saves time. You can program the machine with your desired sequence, and it will build the peptide for you.
Automation and AI speed up the design process for new peptides.
These tools help you avoid errors when developing peptide sequences.
AI can predict the best sequences and check their stability, making your work faster.
Automated systems standardize the process, so you get the same results every time.
You can use these methods for both research and clinical work.
Solid-phase peptide synthesis has changed a lot since automation began in 1965. New machines let you assemble peptide chains quickly and with great control. The Chemputer system can automate every step, even for difficult sequences, and produce very pure peptides.
Method Type | Speed | Accuracy |
|---|---|---|
Rapid and consistent | High reproducibility | |
Manual Techniques | Slower and variable | Prone to errors |
Automated synthesis uses special equipment and computer controls. You get rapid, consistent results. This method lowers the risk of mistakes and ensures your peptides are always high quality.
Note: Automated peptide synthesis methods, such as SPPS, give you faster results and higher accuracy than manual techniques. You can trust the reproducibility of your results.
Protein-Peptide Interactions
You can study how proteins and peptides interact using advanced visualization techniques. Fluorescence microscopy lets you see these interactions in living cells without harming them. Förster resonance energy transfer (FRET) helps you measure how close two proteins are to each other. Fluorescence correlation microscopy (FCM) gives you information about how protein complexes move and how many molecules are present. Bimolecular fluorescence complementation (BiFC) confirms if two proteins interact.
The fluorescent-three-hybrid (F3H) strategy lets you see protein interactions in real time.
You use a nanobody to anchor a GFP-fusion protein, then measure how other proteins interact with it.
Scientists have used this method to watch the p53–HDM2 interaction and see how cancer drugs disrupt it.
You can also use mass spectrometry and other protein analysis techniques to study these interactions. These methods help you find out which proteins interact, how strong the interactions are, and what changes happen during binding. In lab 34, you learn that intrinsically disordered regions (IDRs) and short linear motifs (SLiMs) play a big role in protein-protein interactions. About 40% of amino acids in multicellular organisms are in IDRs. These regions are important for many biological processes and are common in hub proteins.
Traditional techniques like affinity purification mass spectrometry may miss weak interactions, especially those involving SLiMs. You can use advanced techniques like proximity labeling and the PRISMA approach to detect these interactions more effectively.
Callout: You can combine fluorescence microscopy, mass spectrometry, and protein analysis techniques to get a complete picture of protein-peptide interactions. This helps you understand how proteins work together in the cell.
Applications in Medicine and Technology

Targeted Drug Delivery Systems
You can see how peptides and proteins change the way medicine works in the body. Peptide-mediated liposomal systems help drugs reach the right place by using ligand-receptor recognition. These systems increase drug penetrability and accumulation at target sites. Peptide-drug conjugates use cleavable linkers to deliver drugs directly to diseased cells. This method reduces harm to healthy tissues. Antigen peptide mediated polymeric micelles serve as cancer nano-vaccines. They activate the body’s anti-tumor immunity and improve treatment outcomes.
Peptide-mediated liposomal systems boost drug delivery.
Peptide-drug conjugates target diseased cells and protect healthy tissues.
Polymeric micelles act as cancer nano-vaccines.
You may notice that peptide-based delivery systems show strong results in laboratory and animal studies. However, only about 8% of peptide therapeutics get approved for clinical use. This shows that translating these systems to real-world medicine remains a challenge.
Tip: Peptides and proteins offer new ways to deliver drugs, but clinical success rates are still low. You can expect more breakthroughs as research continues.
Peptides as Antibiotics
You can use peptides as antibiotics to fight infections. Antimicrobial peptides have unique mechanisms of action. They target different biological components compared to traditional antibiotics. Many antimicrobial peptides attack the lipid parts of bacterial cell membranes. This leads to direct antimicrobial activity and helps stop infections.
Antimicrobial peptides work against bacteria, viruses, fungi, and parasites.
They can fight antibiotic-resistant organisms by acting on cell membranes.
These peptides modulate immune responses and boost overall activity against infections.
Their ability to use multiple pathways lowers the chance of resistance development.
You can see that peptides provide broad-spectrum activity and help protect human health. Their structure allows them to act quickly and efficiently.
Proteins in Disease
Proteins play a key role in many diseases. When proteins misfold or form aggregates, they lose their normal biological activity. This can lead to serious health problems. You can study the link between protein structure and disease using the table below:
Disease | Key Protein Involved | Type of Aggregation |
|---|---|---|
Parkinson’s disease | α-synuclein | Cytoplasmic aggregates |
Huntington’s disease | Huntingtin | Cytoplasmic aggregates |
Spinocerebellar ataxia | Polyglutamine | Nuclear aggregates |
Alzheimer’s disease | Amyloid-β, tau | Intracellular and extracellular aggregates |
Alzheimer’s disease affects about 10% of adults over 65 in North America. Parkinson’s disease and Huntington’s disease also result from protein misfolding. In these diseases, proteins form aggregates that disrupt normal cell function. For example, in Parkinson’s disease, misfolded α-synuclein leads to Lewy bodies. In Huntington’s disease, a mutation in the huntingtin gene causes protein misfolding and disease progression.
Callout: You can learn how changes in protein structure and activity lead to disease. This knowledge helps you understand the importance of proteins in human health and medicine.
You discovered that a single change can make a protein unfold or refold. You saw how rare amino acids and folding patterns give each protein unique properties. You learned that proteins adapt to their environment and perform more than one function. Lab 34 highlights new protein research:
Novel peptide drug design using machine learning.
Discovery of bioactive peptides from non-coding RNA.
Macrocyclic peptides for protein interactions.
Omics technologies revealing micropeptides.
AI-driven drug discovery for protein interactions.
You can apply your protein knowledge in research projects:
Project Title | Description |
|---|---|
Molecular Dynamics Simulations of Proteins and Nucleic Acids | Simulate protein and nucleic acid interactions to understand their properties. |
Improving the Functionality of Low Protein Streams for High Value Applications | Use protein properties to improve food applications and industry processes. |
You can ask yourself: What new protein discoveries will change medicine or technology next?
FAQ
What is the main difference between a peptide and a protein?
A peptide has fewer than 50 amino acids. A protein contains hundreds or thousands. You can see that proteins have complex shapes and functions, while peptides are usually simpler.
How does temperature affect protein structure?
Heat can cause a protein to unfold. You may notice that cooking an egg changes the protein in the egg white. The protein loses its shape and cannot return to its original form.
Can a protein recover after denaturation?
Sometimes, a protein can refold if you remove the stress gently. For example, cooling a heated protein may help it regain its shape. Strong chemicals often prevent recovery.
Why do rare amino acids matter in protein function?
Rare amino acids give a protein special abilities. Selenocysteine helps protect cells from damage. Hydroxyproline makes collagen strong. You can find these amino acids in proteins with unique roles.
How do scientists study protein interactions in the lab?
You can use fluorescence microscopy to watch protein interactions. Mass spectrometry helps you measure protein binding. These tools let you see how proteins work together in cells.