Antibody knowledge
A Comprehensive Review of Rubella IgG Antibody Testing Solutions

You need a reliable rubella igg antibody testing solution for accurate immunity assessment. ELISA test kits offer high sensitivity and specificity, making them the gold standard for rubella igg antibody detection. Immunoassay analyzers help you process large numbers of samples quickly. Testing for anti-rubella igg matters most for women of childbearing age and during pregnancy. Quantification of rubella virus-specific igg confirms vaccination status and protects against congenital rubella syndrome. Rubella igg antibody testing helps prevent congenital rubella syndrome by detecting immunity before, during, and after pregnancy. You will find that rubella igg antibody assays vary in accuracy, and results can differ between laboratories.
Region | |
|---|---|
European countries | 0.7 – 13.9 |
Pregnant women in some countries | 53 |
Women not targeted by immunization | 6.8 |
A single rubella igg antibody test shows if you have protection against rubella.
Rubella antibody testing is essential for stopping congenital rubella syndrome.
Key Takeaways
Rubella IgG antibody testing is crucial for assessing immunity, especially in women of childbearing age and during pregnancy.
Choose reliable testing methods like ELISA and CLIA for accurate results in rubella immunity assessment.
Regular rubella testing helps prevent congenital rubella syndrome by ensuring mothers are immune before and during pregnancy.
High sensitivity and specificity in tests are essential to avoid false results and ensure proper patient care.
Stay updated on testing standards and new technologies to improve rubella testing efficiency and accuracy.
Rubella IgG Antibody Testing

Reliable Solutions
You need to choose the right rubella IgG antibody testing solution for accurate clinical results. Many laboratories use immunoassay methods to detect anti-rubella virus IgG. These immunoassays include enzyme-linked immunosorbent assay (ELISA) and chemiluminescent immunoassay (CLIA). Both methods help you measure anti-rubella IgG levels in blood samples. Immunoassays offer high sensitivity and specificity, which means you can trust the results for clinical screening and diagnosis.
Immunoassay analyzers process many samples quickly.
Chemiluminescent immunoassay (CLIA) supports diagnosis of rubella virus infection and helps you confirm immunity.
ELISA remains a common choice for serological screening because it is reliable and easy to use.
Some laboratories use both ELISA and CLIA to improve accuracy in clinical settings.
You may see differences in results between immunoassay platforms. These differences can happen because each test uses unique reagents and calibration methods. When you select a testing solution, you should consider the clinical needs of your laboratory. Immunoassay-based rubella IgG antibody testing helps you identify people who have immunity from past infection or vaccination. This is important for clinical screening, especially in women of childbearing age.
Tip: Always check if your immunoassay kit meets international standards for anti-rubella virus IgG detection. This ensures your clinical results are reliable and comparable.
Immunity Assessment
You use rubella IgG antibody testing to assess immunity in different groups. Clinical guidelines recommend serological screening for women before pregnancy. This helps you prevent congenital rubella syndrome. When you test for anti-rubella virus IgG, you can see if a person has protection from rubella. If the IgG level is high, the person is likely immune. If the level is low or absent, you may need to recommend vaccination.
In countries with strong rubella vaccination programs, you may notice that IgG antibody levels decline over time. Even with lower antibody levels, clinical data show that acute rubella and congenital rubella syndrome remain rare. This means vaccination works well, but you still need to screen for anti-rubella IgG to confirm immunity. Some countries, like Finland and Australia, adjust their screening cutoffs based on new clinical data. This helps you make better decisions for patient care.
Serological screening with immunoassay methods gives you a clear picture of immunity in your population. You can use these results to guide vaccination strategies and protect public health. Clinical laboratories rely on anti-rubella virus IgG testing to monitor immunity, especially in women planning pregnancy. Regular screening helps you catch gaps in immunity and prevent outbreaks.
Serological screening identifies people who need vaccination.
Immunoassay-based testing supports clinical decisions for patient care.
Anti-rubella IgG results help you track the success of vaccination programs.
You play a key role in preventing rubella and congenital rubella syndrome by using reliable immunoassay solutions for anti-rubella virus IgG testing. Your clinical expertise ensures that every patient receives the right care at the right time.
Importance of Rubella Testing
Pregnancy and Childbearing Age
Rubella testing plays a vital role in protecting pregnant women and their babies. You need to know if you have immunity to rubella before pregnancy. If you are a healthcare provider, you should always recommend rubella testing for pregnant women. This testing helps you prevent serious health problems for both mother and child.
Pregnant women face high risks if they get rubella during pregnancy. The virus can pass from mother to baby, leading to miscarriage or congenital rubella syndrome. Congenital rubella syndrome can cause heart problems, hearing loss, vision loss, and intellectual disabilities in babies. During the first trimester, rubella can infect all fetal organs. This can result in severe birth defects, death, or abortion. You can see why rubella testing is so important for pregnant women.
Note: Rubella testing is part of the routine prenatal panel for pregnant women. Early detection helps you prevent complications and protect the unborn child.
You use rubella IgG antibody testing for the assessment of immune status in pregnant women. A positive test means the mother has immunity and can protect her baby. If the test is negative, you may recommend vaccination after pregnancy. This step helps prevent future infections in pregnant women.
Vaccination Status
You need to check vaccination status to stop the spread of rubella. Testing helps you see if pregnant women have protection from past vaccination or infection. The assessment of immune status is key for public health and for each patient.
Rubella testing helps you diagnose current or past infection. It also helps you confirm if pregnant women have received the rubella vaccine. The rubella-containing vaccine is safe and effective. One dose protects about 97% of people from rubella. Before the vaccine, congenital rubella syndrome affected up to 4 babies in every 1,000 live births. Now, you can prevent most cases by checking vaccination status and using the assessment of immune status.
Here is a table showing why rubella testing matters for public health and patient care:
Evidence Description | Purpose |
|---|---|
Rubella testing is used to diagnose current or prior infection with the virus. | Helps in identifying infections to control outbreaks and protect vulnerable populations. |
Testing may be performed to determine prior vaccination against rubella. | Assesses immunity, which is crucial for individual patient care and public health. |
Rubella testing is routinely ordered as part of a prenatal panel. | Ensures early detection of infections in pregnant individuals to prevent complications. |
Pregnant individuals infected with rubella can transmit the infection to their fetus. | Highlights the importance of testing to prevent congenital rubella syndrome (CRS). |
The goals of rubella case investigation are to identify infections in pregnant persons. | Aims to prevent exposure of susceptible individuals, thereby reducing the risk of CRS. |
You play a key role in stopping rubella and protecting pregnant women. By checking vaccination status and using rubella IgG antibody testing, you help prevent outbreaks and keep families safe.
Anti-Rubella Virus IgG Testing Methods
Immunoassays
You use immunoassays to measure igg antibodies against rubella. Immunoassays help you detect rubella immunity in blood samples. Most laboratories choose immunoassays because they process many samples quickly and cost less than other methods. Immunoglobulin g immunoassays include ELISA and CLIA. These tests show if you have rubella-specific igg. You can see the strengths and limitations in the table below:
Strengths | Limitations |
|---|---|
High throughput design | Variability in results due to different rubella antigens used by manufacturers |
Relative low cost compared to neutralization assays | Difficulty in interpreting values close to the cut-off |
Ability to detect total levels of rubella-specific IgG | Not all detected IgG may confer protection against rubella infection |
You may notice differences in results between immunoassays. These differences happen because manufacturers use different rubella antigens. You might find that the qualitative result concordance among 14 rubella igg assays was only 56%. The mean coefficient of variation for quantitative results in positive samples reached 51%. High vaccination coverage can also cause false negative results in rubella igg testing.
Hemagglutination Inhibition
You can use the hemagglutination inhibition method to diagnose rubella infection and assess immunity. This method is very sensitive. You mix serum with rubella antigen and red blood cells. If igg antibodies are present, they stop the cells from clumping. You should know that this test faces standardization problems. The type of red blood cells, antigen, and removal of non-specific inhibitors can change the results. You cannot always trust results from a single low dilution of serum. Commercial reagents also vary in reliability.
Tip: Always check the method and reagents used for hemagglutination inhibition to avoid errors in rubella igg detection.
Microneutralization
You use microneutralization to measure rubella igg by testing if antibodies block the virus from infecting cells. This method gives you a direct measure of protective igg. You need special lab equipment and trained staff. Microneutralization takes more time and costs more than immunoassays. You may use this method for research or when you need to confirm immunity.
Western Blot
You use western blot to detect rubella igg with high specificity. This method separates rubella proteins and shows which ones your igg antibodies recognize. Western blot helps you confirm results from other tests. You need skilled staff and special equipment. Western blot takes longer and costs more than immunoassays. You may use it for research or to solve difficult cases.
You have many choices for anti-rubella virus igg testing.
Immunoassays give you fast results for screening.
Hemagglutination inhibition, microneutralization, and western blot help you confirm rubella immunity in special cases.
Standardization Challenges
Calibration Issues
You face many challenges when you compare rubella IgG antibody test results from different laboratories. The lack of standardization in rubella tests leads to variability in results. You may see one test kit show a negative result, while another kit shows a positive result for the same sample. This can make it hard for you to trust the results, especially when you need to assess maternal immune status.
Different test kits use unique reagents and calibration methods.
Poor standardization can cause false-positive or false-negative results.
A false-positive result may give you a false sense of protection against rubella.
A false-negative result could lead to unnecessary vaccination or even decisions to end a pregnancy if rubella exposure occurs.
Rubella vaccination often leads to lower antibody responses compared to wild-type infection. This increases the chance of divergent results across test kits. You must pay close attention to the cutoff levels for IgG, as these differ among manufacturers. These differences can affect how you interpret immune status and make clinical decisions.
Note: Always review the calibration details of your rubella IgG test kit before using it for patient care.
International Standards
International standards help you achieve consistency in rubella IgG antibody testing. The World Health Organization (WHO) created the RUBI-1-94 standard to address assay variation and analytical sensitivity. You use this standard as a reference for calibration across laboratories. This helps you compare results and improve the reliability of rubella testing.
International standards provide a common reference for laboratories.
The RUBI-1-94 standard aims to reduce differences in assay formats and antigen types.
Experts continue to discuss the best cutoff for protective antibody concentration.
Despite these efforts, you may still see variability in results. Different test kits measure various antibodies because the rubella virus has multiple immunogenic antigens. Calibration using the WHO standard has not fully resolved these issues. You need to stay updated on clinical guidelines, as they evolve with new data about protective levels of rubella IgG.
Tip: Following international standards supports the standardization of rubella igg testing and helps you make better decisions for patient care.
You play a key role in ensuring accurate rubella immunity assessment. Reliable testing protects public health and supports safe pregnancy outcomes.
Rubella IgG Assay Comparison
Sensitivity and Specificity
You need to know how well each rubella igg assay performs before you choose a test for your laboratory. Sensitivity tells you how well a test detects people with rubella igg antibodies. Specificity shows you how well a test avoids false positives. High sensitivity and specificity help you trust the results for diagnostics and immunity assessment.
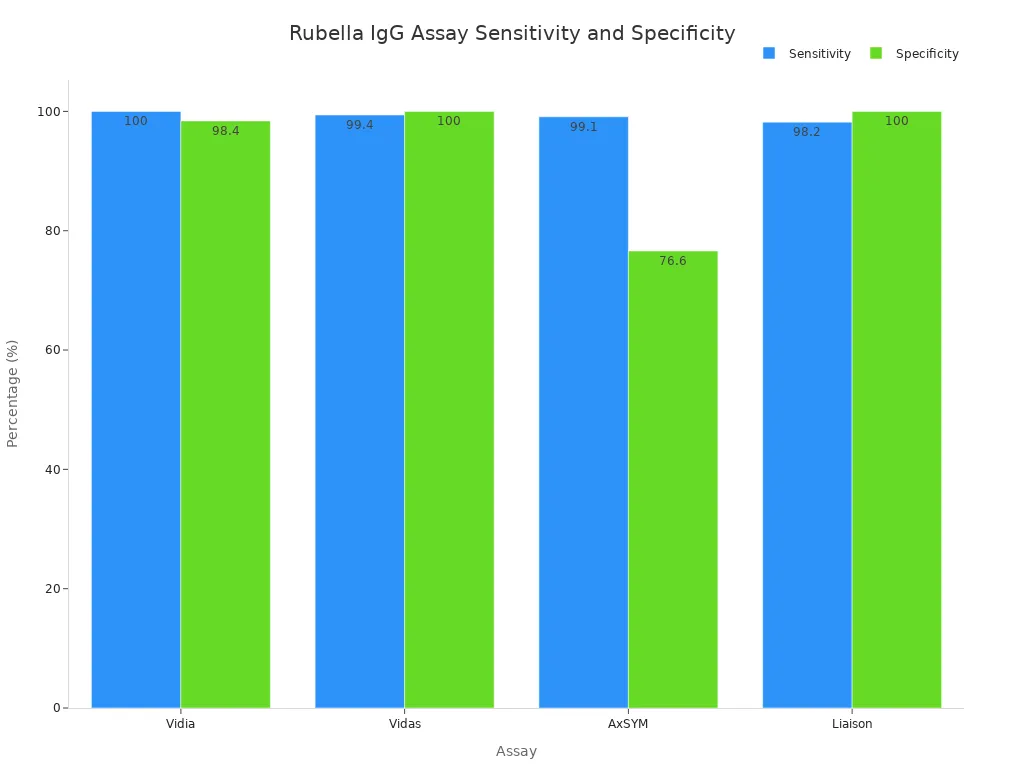
Here is a table that compares the sensitivity and specificity of leading rubella igg assays:
Assay | Sensitivity (%) | Specificity (%) |
|---|---|---|
Vidia | 100 | 98.4 |
Vidas | 99.4 | 100 |
AxSYM | 99.1 | 76.6 |
Liaison | 98.2 | 100 |
RBPG | 95.4 | 100 |
You can see that Vidia and Vidas rubella igg assays have very high sensitivity and specificity. Liaison also shows strong performance for both measures. AxSYM has high sensitivity but lower specificity, which means you may see more false positives with this test. RBPG offers perfect specificity and good sensitivity.
When you select a rubella igg assay, you should look for high sensitivity and specificity. These qualities help you make accurate diagnostic decisions and avoid errors in immunity assessment.
Commercial Assays
You have many commercial rubella igg assays to choose from. Leading brands include Elecsys, LIAISON®, Vidia, Vidas, and AxSYM. Each test uses different technology to measure igg antibodies. Some use enzyme-linked immunosorbent assay (ELISA), while others use chemiluminescent immunoassay (CLIA).
Elecsys rubella igg assay uses electrochemiluminescence for fast and precise results.
LIAISON® rubella igg assay uses CLIA technology and offers high sensitivity and specificity.
Vidia and Vidas rubella igg assays use ELISA and show strong performance in diagnostics.
AxSYM rubella igg assay uses microparticle enzyme immunoassay but may show lower specificity.
You should know that commercial rubella igg assays provide quantitative results. This means you get a number that shows the amount of igg antibodies in the blood. Quantitative determination helps you assess immunity and confirm vaccination status. You can use these tests for both research and diagnostic purposes.
Tip: Always check the package insert for each rubella igg assay. You need to know the cutoff values, calibration method, and recommended specimen type.
You may notice that some commercial assays work better for screening, while others suit confirmatory diagnostics. Elecsys and LIAISON® rubella igg assays are popular for routine diagnostics because they process many samples quickly and give reliable results.
Practical Considerations
You need to think about several factors before you choose a rubella igg assay for your laboratory. The right test helps you make accurate diagnostic decisions and supports public health.
Here is a table that lists practical considerations for rubella igg assay selection:
Consideration | Details |
|---|---|
Testing Purpose | Rubella igg serology testing is essential for assessing immunity to rubella. |
Specimen Collection Guidelines | Guidance on specimen collection, storage, and shipment is provided by CDC. |
Testing Limitations | Rubella IgM testing is not recommended for screening asymptomatic individuals. |
Timing of Testing | Testing should be conducted before, during, and after pregnancy to assess immunity. |
You should always follow specimen collection guidelines to avoid errors in rubella igg assay results. You need to store and ship samples correctly. You should not use rubella IgM tests for screening people without symptoms. Rubella igg assays work best for immunity assessment and diagnostics.
You need to test women before pregnancy, during pregnancy, and after pregnancy. This helps you protect mothers and babies from rubella infection. You should choose a rubella igg assay that fits your laboratory workflow and meets clinical needs.
Note: You play a key role in diagnostics and public health by selecting the right rubella igg assay. Accurate tests help you prevent congenital rubella syndrome and support safe pregnancy outcomes.
You can use commercial rubella igg assays for both research and diagnostic purposes. Quantitative results help you track immunity in your population and guide vaccination strategies. You need to review sensitivity, specificity, and practical considerations before you choose a test.
Selecting Testing Solutions
Key Factors
You need to consider several key factors when you select a rubella IgG antibody testing solution. The clinical context matters most. If you work in a hospital or clinic, you often test women who plan to become pregnant. You want to make sure they have enough antibodies to protect themselves and their babies from rubella. A positive IgG test means vaccination is not needed.
You should look at the sensitivity and specificity of each test. High sensitivity helps you find people who have rubella antibodies. High specificity means you avoid false positives. The table below shows how two common test methods perform:
Test Method | Sensitivity | Specificity |
|---|---|---|
EIA | 94% | 100% |
Latex Agglutination | 94% | 100% |
You also need to think about the overall percent agreement. This metric tells you how often the test results match the true status. For rubella IgG tests, the overall percent agreement can reach 94.4%. You want to choose a test with high agreement to make sure your results are accurate.
Other important factors include:
Need for accurate diagnosis to prevent congenital rubella syndrome
You should always match the test to your patient’s needs. Fast results and easy-to-use kits help you work efficiently.
Research vs. Diagnostics
You may use rubella IgG antibody tests for research or for clinical diagnostics. In research, you often need detailed data about rubella immunity in large groups. You might use tests with high sensitivity to study how rubella spreads or how well vaccines work. You may also compare different test methods to find the best one for your study.
In diagnostics, you focus on individual patients. You want to know if a person has rubella antibodies. You use tests with high specificity to avoid false alarms. You need reliable results to guide vaccination and protect against rubella infection.
You should choose a testing solution that fits your goals. For research, you may select tests that give you more data. For diagnostics, you pick tests that help you make quick and accurate decisions for patient care.
Tip: Always review the test’s sensitivity, specificity, and ease of use before you decide. The right rubella IgG antibody test helps you protect public health and prevent congenital rubella syndrome.
You want the most reliable rubella IgG antibody testing solutions for your patients. Enzyme immunoassays give you the best sensitivity for rubella detection, as shown in the table below:
Testing Method | Reliability Level |
|---|---|
Enzyme Immunoassays (EIAs) | Most commonly used, more sensitive |
Hemagglutination Inhibition (HI) | Previously common, less sensitive |
You should choose enzyme immunoassays for routine rubella screening, especially for women of childbearing age. Standardization helps you compare results across labs. New trends in rubella testing include antigenic microarrays and automation, which will make testing faster and more efficient.
Future Trends in Rubella IgG Testing Solutions | Description |
|---|---|
Antigenic Microarrays | Parallel testing for many pathogens |
Automation of Testing Processes | Faster results, less manual work |
Accurate rubella testing protects mothers and babies. Stay updated on new technologies to improve your lab’s performance.
FAQ
What does a positive rubella IgG test mean?
A positive result shows that you have immunity. You either had a past infection or received the vaccine. You do not need another vaccine if your test is positive.
How often should you get tested for rubella immunity?
You should get tested before pregnancy or if your vaccination status is unknown. Most people do not need regular testing after confirmed immunity.
Can you get rubella from the vaccine?
No, you cannot get the disease from the vaccine. The vaccine uses a weakened virus that cannot cause illness in healthy people.
What should you do if your rubella IgG test is negative?
If your test is negative, you do not have immunity. Your doctor may recommend vaccination to protect you from infection.
Are rubella IgG tests safe for pregnant women?
Yes, the test is safe. It only requires a small blood sample. The test does not harm you or your baby.

