News & Events
Antibody Mediated Rejection Help Without the Hype
You may hear the term antibody mediated rejection if you have had a transplant. This type of rejection happens when your immune system creates antibodies that target the transplanted organ. In lung transplantation, patients with pulmonary capillaritis early after surgery often face poor survival, with only 38% living five years. Many people struggle to clear donor-specific antibodies, which leads to graft problems and faster organ failure. Antibodies play a major role in both lung and kidney transplant rejection. You will find clear, practical guidance here without confusing medical jargon.
- Antibody mediated rejection can lead to chronic lung allograft dysfunction and high mortality rates.
- Patients unable to remove donor-specific antibodies often have worse outcomes.
Key Takeaways
- Antibody mediated rejection can severely impact transplant success. Understanding this process is crucial for protecting your graft.
- Regular monitoring for donor-specific antibodies is essential. Early detection can lead to better treatment outcomes and improved graft survival.
- Plasmapheresis is a key treatment for antibody mediated rejection. It helps remove harmful antibodies from your blood, improving your chances of recovery.
- Stay informed about new therapies and clinical trials. Innovative treatments may offer additional options for managing antibody mediated rejection.
- Maintain open communication with your healthcare team. Ask questions about your risk, treatment options, and monitoring strategies to stay proactive in your care.
Antibody Mediated Rejection Overview
What Is AMR?
You may wonder what antibody mediated rejection means after a transplant. This process happens when your immune system creates antibodies that attack the new organ. These antibodies see the transplanted tissue as foreign and try to remove it. In lung transplantation and renal transplantation, antibody mediated rejection can cause serious problems. Doctors use specific criteria to diagnose this type of rejection. You can see the main criteria in the table below:
| Criteria | Description |
|---|---|
| Histologic evidence of acute tissue injury | You may have signs like glomerulitis, peritubular capillaritis, or acute tubular injury. |
| Histologic evidence of antibody interaction | Doctors look for linear C4d staining or signs of endothelial injury. |
| Detection of DSAs | Blood tests show donor-specific antibodies. |
You might hear your doctor talk about donor-specific antibodies. These antibodies target the organ you received. If you have these antibodies, you face a higher risk of rejection. In lung transplantation, antibody mediated rejection can lead to damage in the blood vessels and tissues. In renal transplantation, the same process can harm the kidney and reduce its function.
Why It Matters
Antibody mediated rejection affects your health and the success of your transplant. You need to know why this matters. When antibodies attack the organ, you may lose graft function. Doctors see this problem often in lung transplantation and renal transplantation. You may face a higher risk of losing your graft if antibody mediated rejection happens.
- Antibody mediated rejection is linked to graft loss in transplant recipients.
- You may have limited treatment options to stop or reverse rejection.
- Doctors look for both clinical and tissue signs of rejection.
- Antibody mediated rejection is common in kidney transplant patients.
- You may face a higher risk of death-censored graft failure.
You should understand that antibodies play a major role in rejection. If you have donor-specific antibodies, you need close monitoring. Early detection and treatment can help protect your graft and improve your outcome.
Mechanisms

Donor-Specific Antibodies
You may hear your doctor talk about donor-specific antibodies after lung transplantation. These antibodies target the organ you received. Donor-specific antibodies act as important markers for antibody mediated rejection. You can have preformed donor-specific antibodies before your transplant, or you may develop new ones after surgery. Preformed donor-specific antibodies can cause hyperacute rejection, which happens very quickly. New donor-specific antibodies often lead to late or chronic antibody mediated rejection. These antibodies can activate the complement pathway, which damages the graft. Some donor-specific antibodies cause injury by triggering antibody-dependent cellular cytotoxicity. This process can lead to chronic rejection and loss of graft function.
- Donor-specific antibodies predict antibody mediated rejection and graft loss.
- Preformed donor-specific antibodies can cause early rejection.
- New donor-specific antibodies are linked to chronic rejection.
- Donor-specific antibodies activate the complement system and damage the graft.
How Damage Occurs
Antibody mediated rejection starts when donor-specific antibodies bind to cells in your transplanted organ. In lung transplantation, these antibodies attack the blood vessels and tissues. The binding of donor-specific antibodies to endothelial cells leads to complement-dependent tissue damage. You may see C4d, a product of the complement cascade, in biopsy samples. Sometimes, antibody mediated rejection happens even without C4d. Activated monocytes and macrophages move into the graft and cause more injury. Donor-specific antibodies can also trigger CD4 T-cells, which add to the damage. Microvascular endothelial cells are the main targets of donor-specific antibodies. Antibodies can change how these cells work, which affects graft function and leads to rejection.
Note: Understanding how donor-specific antibodies interact with endothelial cells helps doctors recognize the signs of antibody mediated rejection in lung transplantation.
Key Cells
You need to know which cells play a role in antibody mediated rejection. T follicular helper cells and B cells help your body make donor-specific antibodies. These cells work together when your immune system sees the transplanted organ as foreign. Plasma cells also produce antibodies that attack the graft. The complement system, which includes many proteins, gets activated by donor-specific antibodies and causes tissue injury. Monocytes and macrophages enter the graft and add to the damage. The combination of these cells and antibodies leads to rejection and loss of graft function in lung transplantation.
- T follicular helper cells and B cells help create donor-specific antibodies.
- Plasma cells produce antibodies that attack the graft.
- The complement system causes tissue injury.
- Monocytes and macrophages add to graft damage.
Diagnosis
Signs and Symptoms
You may not notice any symptoms at first when antibody mediated rejection starts. Many people with lung transplantation experience a drop in their breathing test results, even if they feel fine. Some patients develop mild shortness of breath. Others face severe breathing problems that need urgent care. You should watch for these signs after your transplant.
- Asymptomatic decline in spirometry
- Mild dyspnea
- Fulminant hypoxemic respiratory failure
Doctors also see other symptoms in transplant patients. You may feel short of breath even with little activity. Some people show signs of heart failure. A few patients develop cardiogenic shock, which is very serious.
| Clinical Sign | Number of Patients |
|---|---|
| Dyspnea on minimal exertion | 18 |
| Clinical signs of heart failure | 12 |
| Cardiogenic shock | 6 |
You should report any new symptoms to your doctor. Early detection helps protect your graft and improves your outcome.
Lab Tests
Doctors use several lab tests to check for antibody mediated rejection. These tests help find antibodies that attack your transplanted organ. You may hear about donor-specific anti-HLA antibodies. These antibodies show a higher risk for rejection in both lung transplantation and renal transplantation. Your doctor may also order a test for donor-derived cell-free DNA. This test helps find rejection early, even if you do not have symptoms. It is especially useful for kidney transplant patients.
| Test Type | Description |
|---|---|
| Donor-specific anti-HLA antibodies (DSA) | Key biomarker for assessing the risk of antibody mediated rejection. |
| Donor-derived cell-free DNA (dd-cfDNA) | Used in renal transplantation to exclude subclinical and acute rejection. |
| Histological examination and biopsy | Important for confirming rejection, along with other diagnostic criteria. |
You may need regular blood tests to check for antibodies. Doctors use these results to guide your treatment and monitor your graft.
Biopsy
A biopsy gives doctors a closer look at your transplanted organ. They take a small sample of tissue and check for signs of antibody mediated rejection. You may see terms like C4d staining or endothelial injury in your biopsy report. These findings help confirm rejection. Biopsy remains a key part of diagnosis in lung transplantation and renal transplantation.
Doctors measure how well biopsy works for diagnosis. The positive predictive value (PPV) shows how often a positive result means you have rejection. The negative predictive value (NPV) shows how often a negative result means you do not have rejection.
| Measure | Value (%) |
|---|---|
| PPV (Positive Predictive Value) | 64 |
| NPV (Negative Predictive Value) | 91 |
| PPV for ABMR or mixed rejection | 50 |
| NPV for ABMR or mixed rejection | 94 |
You should know that biopsy is not perfect. Sometimes, you may need more tests to confirm antibody mediated rejection.
Confirmation
Doctors use a combination of signs, lab tests, and biopsy results to confirm antibody mediated rejection. You may need several tests before your doctor makes a final diagnosis. Experts now use new tools and big data to improve accuracy. Recent studies show that donor-derived cell-free DNA and urinary exosome mRNA signatures help diagnose rejection early. These tests are available for kidney transplant patients and may help with lung transplantation in the future.
| Diagnostic Tool | Description | Impact on ABMR Diagnosis |
|---|---|---|
| New assays and molecular tests | Diagnostic and prognostic tools for ABMR | Indicators of active ABMR damage and worse graft outcomes |
| Donor-derived cell-free DNA (dd-cfDNA) | Fragmented extracellular DNA from dying cells | Levels > 1% linked to allograft rejection |
| Urinary exosome mRNA signatures | Noninvasive biomarkers for ABMR | Potential for early diagnosis and management |
Tip: Ask your doctor about new diagnostic tests. These tools may help find rejection before symptoms appear.
Doctors follow consensus guidelines to diagnose antibody mediated rejection. They use a mix of clinical signs, lab results, and biopsy findings. New approaches and big data help doctors make better decisions and protect your graft. You should stay informed about new tests and strategies for lung transplantation and renal transplantation.
Treatment
You may feel overwhelmed when you hear about treatment for antibody mediated rejection. You have several treatment options, and doctors use different strategies depending on your situation. You need to know how each treatment works and what to expect.
Plasmapheresis
Plasmapheresis is a main treatment for antibody mediated rejection. Doctors use this method to remove harmful antibodies from your blood. You may hear about different types of plasmapheresis. Plasma exchange is the most common in the United States. You receive a 1.0 to 1.5 volume exchange, and doctors use albumin to replace your plasma. Double filtration plasmapheresis filters your plasma to take out antibodies. Immunoadsorption plasmapheresis uses special membranes to target and remove antibodies. Sometimes, doctors combine plasmapheresis with other therapies like IVIG, rituximab, mycophenolate, or bortezomib. This combination helps prevent antibody rebound and improves your outcome. The Johns Hopkins Desensitization Protocol uses plasmapheresis every other day, followed by IVIG to lower donor-specific antibody levels.
| Modality | Description |
|---|---|
| Plasma Exchange | Most frequent modality in the US, involves 1.0-1.5 volume exchange using albumin as replacement. |
| Double Filtration Plasmapheresis | A method that filters plasma to remove antibodies. |
| Immunoadsorption Plasmapheresis | Selective modality using adsorbent membranes for antibody elimination. |
| Combination with Other Therapies | Often used with IVIG, rituximab, mycophenolate, and bortezomib to prevent antibody rebound. |
| Johns Hopkins Desensitization Protocol | Every other day PP followed by 100 mg/kg IVIG after each session to decrease DSA levels. |
You may need several sessions of plasmapheresis to lower antibody levels. Doctors use this treatment for both acute humoral rejection and chronic antibody mediated rejection. You should ask your doctor which type of plasmapheresis is best for you.
IVIG
IVIG stands for intravenous immunoglobulin. Doctors use IVIG to help control antibody mediated rejection. IVIG gives your body healthy antibodies that block harmful ones. You may receive IVIG after plasmapheresis to prevent antibody rebound. The VIPAR trial showed that high-dose IVIG helps treat chronic active antibody mediated rejection. Patients in the trial had better tissue health and improved organ function. You may get IVIG as part of your treatment plan for lung transplantation or renal transplantation. IVIG works best when combined with other therapies like plasmapheresis or rituximab.
Tip: IVIG can help lower donor-specific antibodies and protect your graft after acute humoral rejection.
New Therapies
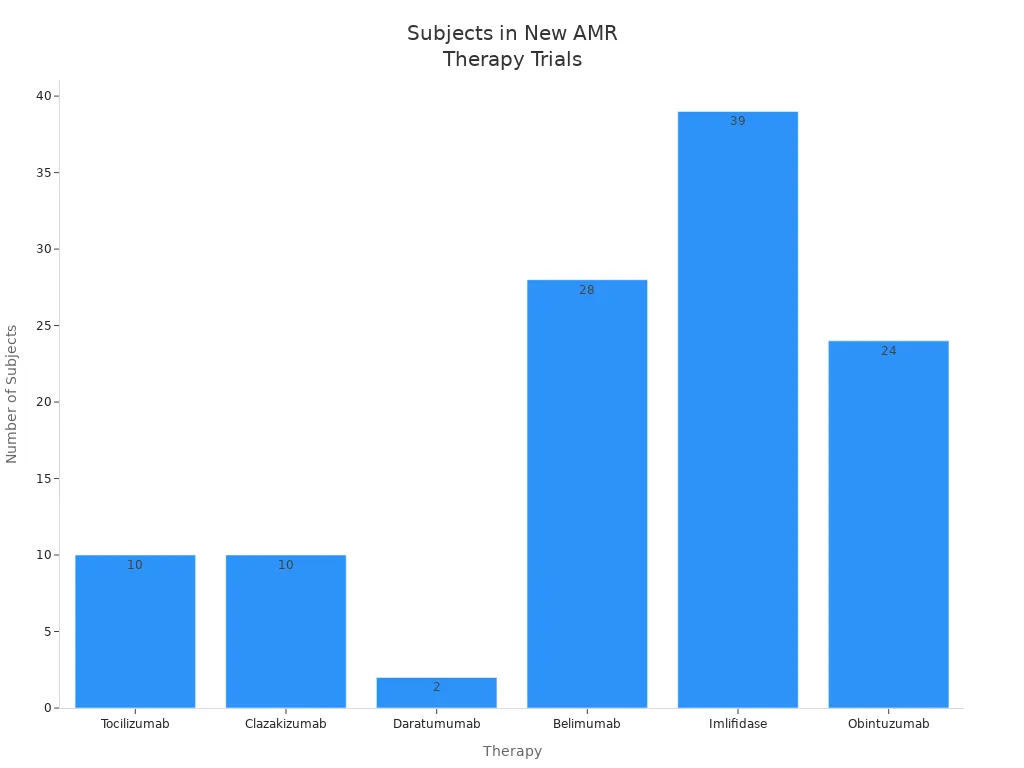
Doctors continue to study new therapies for antibody mediated rejection. You may hear about drugs that target specific parts of your immune system. Tocilizumab blocks the IL-6 receptor and helps with desensitization. Clazakizumab also targets IL-6 and treats refractory or chronic antibody mediated rejection. Daratumumab attacks CD38 on plasma cells and helps lower antibody levels. Belimumab blocks B lymphocyte stimulator and may prevent rejection. Imlifidase is a recombinant enzyme that helps with desensitization and improves graft survival. Obintuzumab targets CD20 and depletes B cells.
| Therapy | Mechanism | Indication | Trial Phase | Subjects | Outcomes | Adverse Events |
|---|---|---|---|---|---|---|
| Tocilizumab | IL-6 receptor inhibitor | Desensitization | Phase I/II clinical trial | 10 | DSA titers, prevention of AMR | Infections, gastrointestinal perforation, elevation of transaminases |
| Clazakizumab | IL-6 inhibitor | Refractory AMR, chronic AMR | Phase II single center open label study | 10 | eGFR, DSA titers, graft inflammation | Diverticulitis, pleural effusion, acute kidney injury |
| Daratumumab | Monoclonal antibody targeting CD38 | Desensitization | Clinical trial | 8 non-human, 2 human | DSA titers | Infusion-related reaction, volume overload, hypogammaglobulinemia, myelosuppression, gastrointestinal upset, infection |
| Belimumab | Anti-B lymphocyte simulator (BLyS) | Prevention of AMR | Phase II clinical trial | 28 | Comparison to standard of care results and infection rates | Gastrointestinal upset, dizziness, infection, depression, diabetes |
| Imlifidase | Recombinant cysteine protease | Desensitization | Multicenter clinical trial | 39 | Graft survival, patient survival, rates of AMR | Well tolerated, safety is currently being studied |
| Obintuzumab | Anti-CD20 | Desensitization | Phase I clinical trial | 24 | Adverse events, B-Cell depletion | Infections, thrombocytopenia, infusion-related reactions, cardiac events |
You may see these new therapies in clinical trials for lung transplantation and renal transplantation. Doctors hope these drugs will improve outcomes and reduce rejection rates.
Note: Ongoing research explores monoclonal antibodies like rituximab, plasma cell depletion with bortezomib, and complement inhibition using eculizumab. New agents such as belimumab and epratuzumab may help suppress B cells and prevent acute humoral rejection.
What to Expect
You may wonder what results you can expect from treatment for antibody mediated rejection. Doctors look for short-term and long-term outcomes. You may see improvement in kidney function, with serum creatinine dropping below 1.5 mg/dl after treatment. Lower donor-specific antibody levels mean less risk of graft loss. Doctors use clinical assays and new monitoring tools to track your progress. Over time, better kidney function leads to improved allograft survival. Longitudinal changes in DISPO scores help doctors predict failure risk.
| Outcome Type | Short-term Outcomes | Long-term Outcomes |
|---|---|---|
| Kidney Function | Improvement in serum creatinine to < 1.5 mg/dl | Better allograft survival associated with improved function |
| DSA Levels | Decrease in DSA MFI values after treatment | Lower odds of allograft loss |
| Monitoring Tools | Use of clinical assays and potential novel approaches | Longitudinal changes in DISPO scores linked to failure risk |
Doctors use different treatment strategies for acute humoral rejection and chronic antibody mediated rejection. For acute humoral rejection, you may receive plasmapheresis, IVIG, steroids, and sometimes rituximab. Chronic antibody mediated rejection often needs supportive care and optimized immunosuppressive therapies.
| Type of Rejection | Recommended Treatment Strategies |
|---|---|
| Acute AMR | Plasmapheresis, IVIG, Steroids, ± Rituximab |
| Chronic AMR | Supportive care, Optimized baseline immunosuppression |
You may need regular follow-up and monitoring after treatment. Doctors check for new antibodies and watch for acute rejection episodes. You should stay informed about new treatment options and research. Early and effective treatment helps protect your graft and improves your quality of life after lung transplantation or renal transplantation.
- Ongoing research focuses on new ways to treat antibody mediated rejection:
- Monoclonal antibodies like rituximab target B cells.
- Plasma cell depletion with bortezomib helps lower antibody levels.
- Complement inhibition with eculizumab blocks tissue damage.
- New agents such as belimumab and epratuzumab may help suppress B cells.
Callout: Ask your doctor about new therapies and clinical trials. You may benefit from innovative treatments for antibody mediated rejection.
Renal Transplantation and AMR
AMR in Kidneys
You face unique challenges with antibody mediated rejection after renal transplantation. This type of rejection causes more problems in kidney transplants than in other organs, such as lung transplantation. Antibody mediated rejection leads to both acute and chronic graft dysfunction. You may lose your graft faster if donor-specific antibodies appear. Doctors first noticed the harmful effects of antibodies on kidney grafts in the 1960s. Since then, experts have learned that antibodies play a major role in rejection and poor graft survival. If you have donor-specific antibodies, you have a higher risk of losing your kidney graft. Antibody mediated rejection remains the leading cause of graft loss in renal transplantation. You need to understand how antibodies attack the kidney and why monitoring is so important.
Note: Donor-specific antibodies can cause immediate failure of a kidney graft. You should ask your doctor about your antibody levels after renal transplantation.
Monitoring
You need regular monitoring to protect your graft and improve graft survival after renal transplantation. Doctors use several strategies to check for antibody mediated rejection. You should watch your kidney function closely. If your estimated glomerular filtration rate (eGFR) returns to within 10% of your baseline, you have a better chance of long-term graft survival. Doctors also track donor-specific antibodies. If your antibody levels drop by more than 50%, your risk of rejection goes down. Proteinuria, or protein in your urine, is another sign. If your proteinuria improves by over 25%, you have a better outlook for graft survival.
- Monitor eGFR and look for changes near your baseline.
- Check donor-specific antibodies regularly.
- Watch for improvements in proteinuria.
Doctors need better ways to detect chronic active antibody mediated rejection early. You should ask about new tests and monitoring tools. Early detection helps you keep your graft healthy and improves your chances for long-term graft survival.
| Monitoring Strategy | Benefit for Graft Survival |
|---|---|
| eGFR within 10% of baseline | Best predictor of long-term survival |
| DSA decline > 50% | Lower risk of rejection |
| Proteinuria improvement >25% | Better graft outcomes |
Tip: Stay in close contact with your transplant team. Early monitoring and treatment help you avoid rejection and keep your kidney graft working well.
Practical Tips
Follow-Up
You need regular follow-up after lung transplantation to protect your graft and lower the risk of antibody mediated rejection. Doctors recommend early protocol biopsies, especially if you have donor-specific antibodies. These biopsies help find rejection before symptoms appear. If your doctor detects antibody mediated rejection early, you have a better chance to save your graft. You may need treatment with corticosteroids, plasmapheresis, IVIG, and rituximab. Doctors often use five sessions of plasmapheresis, IVIG every two sessions, and two doses of rituximab. After treatment, you will have follow-up biopsies to check your response.
- Schedule early protocol biopsies if you have donor-specific antibodies.
- Complete all recommended treatment sessions.
- Attend follow-up appointments for biopsies and lab tests.
Questions for Doctors
You should ask your doctor clear questions about antibody mediated rejection and lung transplantation. Good communication helps you understand your risks and treatment options.
- What is my risk for antibody mediated rejection?
- How often should I have biopsies or lab tests?
- What treatment strategies will you use if you find rejection?
- How do you monitor my graft health?
- What signs should I watch for at home?
Managing Side Effects
Treatments for antibody mediated rejection can cause side effects. Plasmapheresis lowers antibody levels but may lead to infections, bleeding, or allergic reactions. You might also experience hypovolemia or hypocalcemia. Doctors use albumin as a replacement fluid to reduce these risks. You need careful monitoring during treatment.
- Watch for signs of infection or bleeding.
- Tell your doctor about any allergic reactions.
- Drink fluids as advised to prevent hypovolemia.
- Ask about replacement fluids and anticoagulant risks.
Staying Healthy
You can use simple strategies to stay healthy after lung transplantation and reduce rejection risk. Take your medicines as prescribed. Eat a balanced diet and stay active. Avoid people who are sick to lower your infection risk. Keep all follow-up visits and report new symptoms quickly.
Tip: Staying in close contact with your transplant team helps you protect your graft and manage antibodies.
Understanding antibody mediated rejection helps you protect your graft and improve your health. Early diagnosis matters. The table below shows how the timing of antibodies affects rejection and survival:
| Evidence Description | Findings |
|---|---|
| Early DSA Presence | Associated with a 3.3-fold higher risk of ABMR |
| Late DSA Presence | Linked to lower allograft survival and ABMR-free survival |
You should talk with your healthcare team often. They may discuss your case in meetings, share updates, and involve you in treatment choices. Support is available through therapies, NKF Peers, and kidney communities. Stay informed, ask questions, and reach out for help when you need it.
FAQ
What are donor-specific antibodies (DSA)?
Donor-specific antibodies are proteins your immune system makes that target your transplanted organ. Doctors check your blood for these antibodies. High levels can mean a higher risk of rejection.
How often should you get tested for antibody mediated rejection?
You should get regular blood tests and biopsies after your transplant. Doctors may recommend testing every few months, especially if you have donor-specific antibodies or changes in organ function.
Can antibody mediated rejection be cured?
Doctors can treat antibody mediated rejection with therapies like plasmapheresis and IVIG. These treatments lower antibody levels and help protect your graft. Early treatment gives you the best chance for recovery.
What symptoms should you watch for after a transplant?
Watch for shortness of breath, swelling, or changes in urine. You may also notice fatigue or fever. Report any new symptoms to your doctor right away.
Are new treatments available for antibody mediated rejection?
Researchers study new drugs like tocilizumab and daratumumab. These therapies target specific immune cells. Ask your doctor about clinical trials and new options for your condition.