News & Events
Emerging Trends in the Development of Therapeutic Monoclonal Antibody Products
Recent breakthroughs in the development of therapeutic monoclonal antibody products drive remarkable progress for patients and the biopharma industry. In 2024, the market size reached $263.28 billion, with projections showing rapid growth.
| Year | Market Size (USD Billion) | CAGR (%) |
|---|---|---|
| 2023 | 230.87 | N/A |
| 2024 | 263.28 | N/A |
| 2032 | 792.37 | 14.8 |
New therapies such as Bentracimab, Clesrovimab, and Telisotuzumab vedotin highlight how innovation in therapeutic antibodies improves care and expands clinical possibilities. Researchers, clinicians, and industry professionals see these advances as essential for transforming patient outcomes.
Key Takeaways
- Next-generation monoclonal antibodies use advanced techniques to improve patient safety and treatment effectiveness.
- Bispecific and multispecific antibodies enhance selectivity, leading to better treatment outcomes for complex diseases.
- Antibody-drug conjugates combine targeted therapy with cytotoxic drugs, improving efficacy while reducing side effects.
- Ongoing advancements in antibody engineering promise safer and more effective therapies for a wide range of diseases.
- Staying informed about these trends can empower patients and healthcare professionals to make better treatment choices.
Emerging Trends

Next-Generation Antibodies
Scientists continue to develop next-generation mab products using advanced technology. These new therapeutic monoclonal antibodies use humanization techniques to reduce immune reactions in patients. Researchers use Fc engineering to change the Fc region of the antibody, which can improve how long the product stays in the body and how well it works. Phage display allows scientists to screen billions of antibody variants, helping them find the best candidates for therapy. AI-driven platforms now predict how likely an antibody will cause an immune response and help improve its design.
Next-generation mab products address several challenges:
- Codon optimization and host cell engineering increase expression yields.
- CDR grafting and computational modeling help keep strong binding after humanization.
- Fc engineering and glycoengineering reduce unwanted activity.
- Epitope prediction and humanization lower the risk of immune reactions.
Researchers also use single-domain antibodies, called nanobodies, and engineered antibody fragments. These smaller mab products can reach targets that regular monoclonal antibodies cannot. The field now expands beyond cancer and immune disorders, with new research targeting diseases like influenza and HIV.
Bispecific and Multispecific Formats
Bispecific and multispecific mab products represent a major step forward in therapeutic monoclonal antibodies. These antibodies can bind to two or more different targets at the same time. This feature increases selectivity and can improve treatment results.
| Advantage | Bispecific/Multispecific Antibodies | Monospecific Antibodies |
|---|---|---|
| Selectivity | Increased selectivity | Lower selectivity |
| Efficacy | Higher efficacy | Standard efficacy |
| Tolerability | Better tolerability | Variable tolerability |
Bispecific antibodies work well for therapies that need high specificity, such as antibody-drug conjugates and CAR T-cell therapy. Multispecific antibodies offer even more selectivity and better tolerability. They do not require lymphodepletion before infusion and are available off-the-shelf, unlike CAR-T cells. Patients treated with multispecific mab products have a lower risk of cytokine-release syndrome and neurotoxicity.
Recent approvals show the success of these formats:
| Date | Product Name | Type | Indication |
|---|---|---|---|
| June 2022 | Cadonilimab | PD-1/CTLA-4 bispecific | Recurrent or metastatic cervical cancer |
| 2022 | VABYSMO | Bispecific antibody | Oncology (various) |
| 2022 | TECVAYLI | Bispecific antibody | Oncology (multiple myeloma) |
| 2022 | LUNSUMIO | Bispecific antibody | Oncology (diffuse large B-cell lymphoma) |
| 2022 | KIMMTRAK | Bispecific molecule | Oncology (hematological malignancies) |
The FDA has authorized ten bispecific antibodies and one bispecific molecule, mostly for cancer treatment, including multiple myeloma and diffuse large B-cell lymphoma.
Antibody-Drug Conjugates
Antibody-drug conjugates (ADCs) combine the targeting power of mab products with the strength of cytotoxic drugs. These therapeutic monoclonal antibodies deliver drugs directly to cancer cells, which can improve results and reduce side effects. Recent innovations in ADC design, payload selection, and engineering have made these mab products more effective.
- Structural optimization, such as antibody engineering and linker chemistry, improves the therapeutic index.
- New designs, like bispecific ADCs and immunostimulatory ADCs, help overcome tumor heterogeneity and drug resistance.
- Conditioned activated prodrug linkers lower systemic toxicity.
- Degradative-antibody conjugates (DACs) combine ADCs with protein degraders for targeted therapy.
Recent studies show that ADCs can offer higher response rates and manageable side effects compared to other monoclonal therapies.
| Study | Treatment | Efficacy | Safety Profile |
|---|---|---|---|
| Phase I/II | Sacituzumab govitecan + Pembrolizumab | Higher overall response rate in TNBC | Manageable adverse effects (diarrhea, neutropenia) |
| Phase II | Enfortumab vedotin + Pembrolizumab | Improved PFS and ORR in UC | Manageable side effects (rash, fatigue, neuropathy) |
Engineered Efficacy
Researchers use several engineering strategies to improve the efficacy of mab products. Fc engineering changes the Fc region to boost stability and therapeutic properties. Bispecific antibodies allow one mab product to target two different antigens, which can increase the effectiveness of treatment. ADCs combine the selectivity of antibodies with the power of cytotoxic drugs, making them strong tools against cancer.
Scientists also use rational mutagenesis to improve the stability and solubility of monoclonal antibodies. These improvements help make mab products safer and more effective for patients. The integration of advanced genetic engineering, hybridoma technology, and phage display continues to drive progress in the field of therapeutic monoclonal antibodies.
Key advancements in engineered efficacy:
- Fc engineering for better stability and function
- Bispecific and multispecific formats for higher selectivity
- ADCs for targeted cancer therapy
- Rational mutagenesis for improved stability
The development of these new mab products opens more possibilities for treating a wide range of diseases, from cancer to infectious diseases and immune disorders.
Monoclonal Antibodies Overview
What Are Monoclonal Antibody Products
Monoclonal antibody products are laboratory-made molecules that act like natural antibodies in the body. Scientists design these products to target specific proteins or cells. They use advanced biotechnology to produce monoclonal antibody products with high precision.
Researchers use several methods to create these antibodies:
- Hybridoma technology uses animals to produce B lymphocytes. Scientists fuse these cells with myeloma cells to make hybridomas. Hybridomas produce specific monoclonal antibodies.
- Phage display technology uses phage libraries. Scientists display antibody fragments on phages and select the best candidates.
- Single B-cell technology isolates individual B-cells. This method allows rapid production of highly specific monoclonal antibody products.
These production methods help scientists develop antibodies for many diseases. Each technique improves the speed and accuracy of antibody discovery.
Clinical Significance
Monoclonal antibody products play a major role in modern medicine. Doctors use these products to treat many conditions. Clinical trials test monoclonal antibody products for safety and effectiveness.
The table below shows the most common clinical indications for monoclonal antibody products:
| Clinical Indication | Percentage (%) | Count |
|---|---|---|
| Hyperlipidemia | 39.1 | 136 |
| Prophylaxis of respiratory syncytial virus infection | 29.9 | 104 |
| Osteoporosis | 12.1 | 42 |
| Atopic dermatitis | 4.6 | 16 |
| Nasal polyposis | 3.4 | 12 |
| Osteoarthritis, sinusitis | 2 | 7 |
| Urticaria | 1.1 | 4 |
| Rheumatoid arthritis | 0.9 | 3 |
| Asthma | 0.9 | 3 |
| Migraine | 0.3 | 1 |
| Bilateral sacroiliitis | 0.3 | 1 |
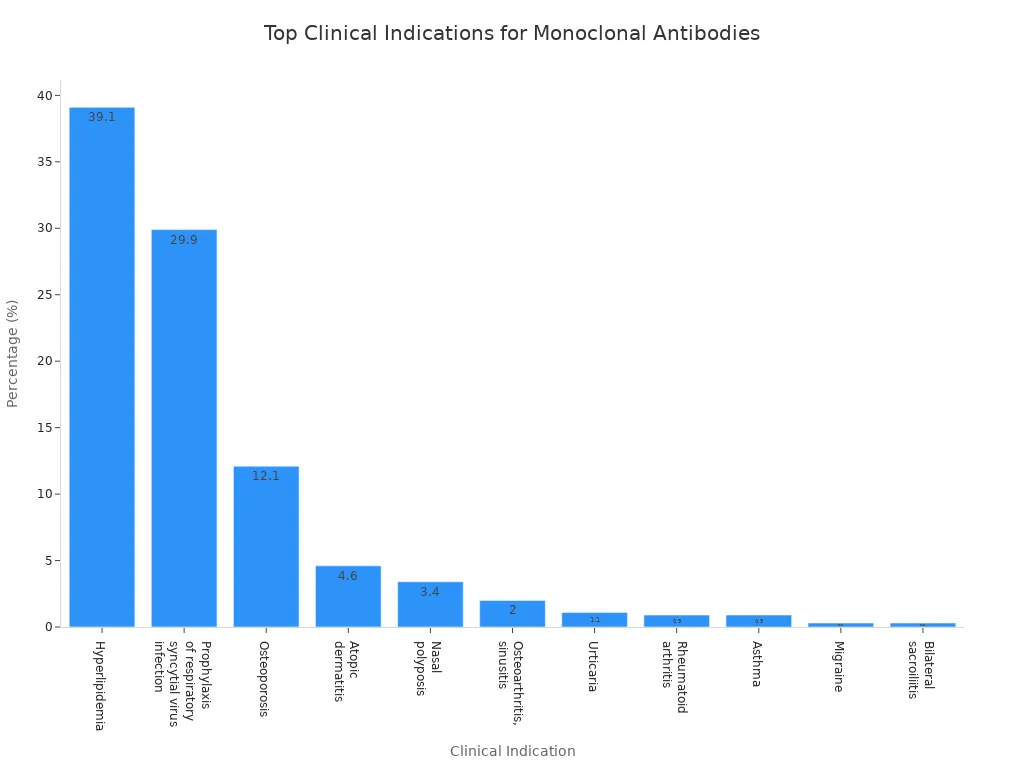
Doctors rely on monoclonal antibody products for conditions like hyperlipidemia, osteoporosis, and respiratory infections. Clinical trials continue to expand the use of these antibodies. Monoclonal antibody products improve patient outcomes and offer new hope for many diseases.
The Development of Therapeutic Monoclonal Antibody Products
Discovery and Engineering
The development of therapeutic monoclonal antibody products began in the 1970s. Scientists generated the first monoclonal antibody in 1975. The first fully licensed therapeutic monoclonal antibody product appeared in 1986. After this milestone, research activity increased quickly. The introduction of chimeric monoclonal antibody products in the 1990s, such as ReoPro® (abciximab), improved patient outcomes by reducing anti-drug antibodies.
Over 2000 research and development programs have started since 1985. Notable increases in research followed major technological advances in 1987 and 1999.
The hybridoma technique plays a key role in the discovery of mab products. Scientists use this method to fuse a single B cell with a myeloma cell. This fusion creates a hybridoma that produces large amounts of a specific monoclonal antibody. The hybridoma technique allows for high specificity, large-scale production, and long-term preservation of monoclonal antibody products.
| Contribution | Description |
|---|---|
| Specificity | Produces highly specific monoclonal antibody products from a single B cell. |
| Quantity | Enables large-scale production of mab products. |
| Applications | Used in diagnostics and cancer therapy. |
| Preservation | Hybridoma cell lines can be stored long-term. |
Scientists also use genetic engineering and phage display to improve mab products. These methods help select the best monoclonal antibody products for therapeutic use.
Preclinical to IND
The development of therapeutic monoclonal antibody products follows a clear path from discovery to clinical testing. The main stages include:
- Target discovery and validation
- Antibody discovery
- Characterization and lead selection
- Engineering and optimization
- Candidate selection and cell line development
After these steps, researchers test mab products in preclinical studies. These studies check safety and effectiveness in the lab and in animals. If results look promising, scientists prepare an Investigational New Drug (IND) application. The IND filing allows clinical trials in humans to begin.
The global value of monoclonal antibody products now reaches about $20 billion each year. This growth shows the importance of the development of therapeutic monoclonal antibody products for medicine and patient care. Advances in discovery, engineering, and preclinical testing continue to drive new therapeutic options for many diseases.
Market Dynamics
Growth and Investment
The market for monoclonal antibody products continues to grow rapidly. Companies invest in every stage, including discovery, development, manufacturing, and distribution. Mab products now play a key role in treating cancer, autoimmune diseases, and infectious diseases. The market also covers services like research and development, clinical and commercial biologics manufacturing, and cold-chain distribution.
- The monoclonal antibody therapeutics market reached $222.6 billion in 2023.
- Projections show the market will grow to $497.5 billion by 2029.
- The expected compound annual growth rate is 14.5% from 2024 to 2029.
- Drivers include more complex diseases, better biotechnology, and faster regulatory approvals.
Advanced antibody engineering and precision targeting improve therapeutic efficacy. Innovations such as bispecific antibodies and antibody-drug conjugates change how doctors treat diseases, especially in oncology. These advancements help expand the range of treatable conditions and improve patient outcomes.
Recent Approvals
Regulatory agencies around the world continue to approve new mab products. These approvals show the progress in therapeutic monoclonal antibody products and highlight the variety of diseases they can treat.
| Product Name | Trade Name | Target | Type | Indication(s) | Regulatory Agency | Year |
|---|---|---|---|---|---|---|
| Tixagevimab + Cilgavimab | Evusheld | SARS-Cov-2 spike protein | Canonical antibody | Severe acute respiratory syndrome coronavirus 2 prophylaxis | EMA Europe | 2022 |
| Serplulimab | Hansizhuang | Programmed cell death 1 (PD-1) | Canonical antibody | Non-small cell lung cancer, Small cell lung cancer, Solid cancer | NMPA China | 2022 |
| Tebentafusp | Kimmtrak | CD3, Glycoprotein 100 | Immunoconjugate | Uveal melanoma | FDA US | 2022 |
These new monoclonal antibody products address urgent medical needs. They also show how mab products can target a wide range of diseases, from viral infections to cancer.
Global Expansion
Access to monoclonal antibody products is increasing worldwide. However, most sales still occur in high-income regions. The following table shows the distribution of mab products sales compared to the global population:
| Region | Percentage of mAb Sales | Percentage of Global Population |
|---|---|---|
| U.S., Canada, Europe | 80% | 15% |
| Low- and Middle-Income Countries | 20% | 85% |
Mab products reach more patients each year, but low- and middle-income countries still face challenges in access. Companies and health organizations work to close this gap by expanding manufacturing and distribution networks. As global expansion continues, more people benefit from therapeutic advances in monoclonal antibody products.
Drivers of Change
Technology Advances
New technology shapes the future of monoclonal therapies. Scientists use single B-cell technology to find better mab products. Transgenic animals help create human-like monoclonal treatments. Cell-free protein synthesis speeds up production. Many labs now use single-use bioreactors and improved purification tools. Automation and digitalization make the process faster and more reliable. Hybridoma technology, phage display, and recombinant DNA technology also play important roles. These advances help researchers discover and produce new mab products quickly.
- Single B-cell technology
- Transgenic animals technology
- Cell-free protein synthesis
- Single-use bioreactors
- Improved purification tools
- Automation and digitalization
- Hybridoma and phage display
These tools allow scientists to design mab products that target diseases more precisely. As a result, patients receive safer and more effective monoclonal therapies.
Regulatory Shifts
Regulatory agencies have changed how they review and approve monoclonal therapies. They now focus more on post-marketing surveillance. This means they watch mab products closely after approval to ensure safety and effectiveness. Agencies also require more data when companies want to change dosing. Manufacturers must submit extra applications for any updates to dosing recommendations. These steps keep the standards for monoclonal therapies high and protect patients.
Regulators now demand strong scientific evidence for every change in mab products.
Biosimilars Impact
Biosimilars have changed the market for monoclonal therapies. They make mab products more affordable and easier to access. When biosimilars enter the market, prices drop. For example, the price of trastuzumab fell by $438, infliximab by $112, and bevacizumab by $110. Annual price reductions continue for other mab products. The Essential Medicines List now includes biosimilars, which helps more patients get treatment. Countries like Brazil, India, and South Africa have used biosimilars to treat breast cancer and lymphoma.
- Lower prices for monoclonal therapies
- More patients can access mab products
- Public-private partnerships help expand reach
Biosimilars encourage competition and drive down costs. This change helps health systems and patients around the world.
Manufacturing Evolution in Monoclonal Antibody Products

Process Innovations
Manufacturing processes for monoclonal therapies have changed greatly since 1986. The first licensed monoclonal antibody marked the start of large-scale production. Over time, scientists improved cell culture methods, which led to higher product titers and better yields. Today, advances in mammalian cell culture allow titers to reach more than 5 grams per liter. This means companies can produce more mab products with less waste.
Researchers use platform approaches to streamline downstream processing. High-throughput devices help optimize bioprocesses quickly. Disposable systems and continuous upstream processing make production faster and safer. Continuous chromatography and integrated continuous bioprocessing reduce costs and improve efficiency. Quality by Design and process analytical technologies help ensure that each batch of monoclonal therapies meets strict standards.
Since 1986, more than 70 monoclonal therapies have received approval for therapeutic or diagnostic use. Mab products now make up over half of the biotherapeutic market, with many new antibodies in development.
Laboratory production uses small-scale bioreactors for early-stage research. Plant-based production is gaining interest because it can lower costs and increase scalability. These innovations help companies meet the growing demand for monoclonal therapies.
Supply Chain
The supply chain for mab products requires careful planning and advanced technology. Safe and aseptic filling prevents contamination during packaging. Precise freezing methods protect the structure of monoclonal therapies during cooling. Temperature-controlled storage and transport keep mab products stable and effective.
| Strategy | Description |
|---|---|
| Risk Assessments | Identifies high-risk activities for timely interventions. |
| Validation Master Plan (VMP) | Outlines validation steps to ensure compliance and safety. |
| HAZOP Methodology | Analyzes risks based on severity, likelihood, and detection. |
| Specialized Packaging | Maintains integrity and safety during transport. |
Primary packaging affects the quality and safety of monoclonal therapies. Secondary packaging helps prevent product loss and keeps mab products secure during shipping. Companies use advanced packaging solutions to maintain product integrity from the factory to the patient.
Challenges and Opportunities
Market Access
Many patients face barriers when trying to access new monoclonal antibody products. Several factors limit the reach of these therapies:
- Regulatory barriers slow down approvals.
- Pricing and demand issues affect availability.
- Manufacturing challenges can delay supply.
- Supply chain limitations restrict distribution.
- Licensing complications create legal hurdles.
- Health system and clinical research capacity may be lacking.
Other obstacles include complex manufacturing processes, strict regulatory steps, and intellectual property rights. Some companies lack incentives to expand access, and substitution with other products is often impossible. The innovator’s reach also determines how widely mab products become available.
Cost and Affordability
Cost remains a major challenge for patients who need therapeutic monoclonal antibody products. High prices can limit access, especially in low- and middle-income countries. Recent changes have improved affordability and availability. The table below shows how affordability has changed:
| Metric | Before NMR | After NMR | Change |
|---|---|---|---|
| Average availability of 13 mAbs | N/A | 8.1% | Increased |
| Median unit price of 10 mAbs | N/A | 34.3% | Decreased |
| Average affordability (days of income) | 680 days | 298 days | 56.2% reduction |
These improvements mean more patients can receive needed treatments. Lower prices and better access help health systems provide care to more people.
Meeting Patient Needs
Researchers and manufacturers use several strategies to better meet patient needs with therapeutic monoclonal antibody products. They focus on quality, consistency, and safety during production. The table below highlights some key strategies:
| Strategy | Description |
|---|---|
| Tech Transfer | Define acceptance criteria and validation protocols for quality scale-up. |
| Scaling Up | Maintain consistency and optimize yield from clinical to commercial stages. |
| Purification | Use multiple chromatography steps for high recovery and impurity clearance. |
| Formulation | Ensure stability and sterility with careful buffer and container choices. |
| Analytical Method Transfer | Validate methods for robustness and reproducibility at new sites. |
Teams also assess potency, pharmacokinetics, immunogenicity, and safety to understand patient impact. Flexible specifications help maintain a steady supply while ensuring product quality. New approaches, such as tumor-selective vasculature permeability enhancers and pH-sensitive antibodies, further improve therapeutic outcomes.
Future Outlook
Scientific Breakthroughs
Researchers expect several new technologies to change the future of monoclonal antibody development. These advances will help scientists create more effective treatments for many diseases. The table below shows some of the most promising breakthroughs:
| Breakthrough Technology | Description |
|---|---|
| Bispecific Antibodies | Recognize two distinct antigens, useful in treating cancers and hemophilia. |
| Catalytic Agents | Stereo-specific antibodies that can degrade antigens, showing promise in infection eradication. |
| Antibody-Drug Conjugates (ADCs) | Combine antibody targeting with cytotoxic drugs, improving precision in cancer treatment. |
| Fc Engineering | Modifies antibodies to enhance immune interactions and boost therapeutic efficacy. |
| Next-Generation Sequencing | Analyzes antibody repertoires, helping identify potent new antibodies. |
| Novel Targets | Includes immune checkpoint inhibitors and new cancer antigens, expanding treatment possibilities. |
Scientists believe these breakthroughs will lead to safer and more powerful therapies. New methods like next-generation sequencing allow faster discovery of antibodies that work better for patients.
Market and Care Shifts
The biopharma market continues to change how doctors treat patients with monoclonal antibodies. Several trends shape the future of patient care:
- The rise of monoclonal antibodies provides precision treatments for many diseases.
- Engineered proteins mimic natural immune responses, leading to targeted therapies with fewer side effects.
- More patients receive monoclonal antibodies for cancer and autoimmune diseases.
- The market grows quickly because people want targeted therapies.
- New technology in antibody engineering improves how well treatments work.
- Doctors expect more personalized and effective options for patients.
- Chronic and autoimmune diseases drive the need for new monoclonal antibody therapies.
- Novel approaches focus treatment directly on specific immune system cells.
These changes help patients get better results and fewer unwanted effects. The future promises more choices and improved care for people with serious illnesses.
Long-Term Risks
Monoclonal antibody therapies offer many benefits, but long-term risks remain. Scientists and doctors watch for possible problems that could affect patients over time. Some risks include:
- Immune system reactions that may cause side effects.
- Resistance to therapy as diseases change or adapt.
- High costs that limit access for some patients.
- Supply chain challenges that may disrupt treatment availability.
- Unknown effects from new technologies and engineered antibodies.
Doctors and researchers continue to study these risks to keep patients safe. They use careful monitoring and new safety measures to reduce problems. The future of monoclonal antibody therapies depends on balancing innovation with patient safety.
- Next-generation antibodies, bispecific formats, and antibody-drug conjugates lead innovation.
- Engineered efficacy improves patient outcomes and expands treatment options.
- Researchers and clinicians face new opportunities and challenges with therapeutic monoclonal antibody products.
The future promises safer, more effective therapies. Readers should stay informed and support ongoing research.
FAQ
What are monoclonal antibody products used for?
Doctors use monoclonal antibody products to treat cancer, autoimmune diseases, and infections. These therapies help target harmful cells or proteins. Patients often see better results and fewer side effects compared to older treatments.
How do bispecific antibodies work?
Bispecific antibodies bind to two different targets at once. This design helps doctors treat complex diseases like cancer. Patients may benefit from higher selectivity and improved safety.
Are antibody-drug conjugates safe?
Researchers test antibody-drug conjugates in clinical trials. Most patients experience manageable side effects, such as fatigue or mild rash. Doctors monitor patients closely to keep treatments safe.
Why do monoclonal antibody therapies cost so much?
Manufacturing monoclonal antibody therapies requires advanced technology and strict quality control. These factors increase costs. Biosimilars help lower prices and improve access for more patients.
Can children receive monoclonal antibody treatments?
Doctors prescribe monoclonal antibody treatments for some children with specific diseases. Each therapy must pass safety tests before use in young patients. Pediatric dosing often differs from adult dosing.