News & Events
The Science Behind Immuno Oncology and Immune System Activation
Immuno oncology uses your immune system to fight cancer. You see millions of new cancer cases each year, with numbers rising fast.
- In 2022, doctors diagnosed about 20 million people worldwide.
- Experts predict this number will reach 35 million by 2050.
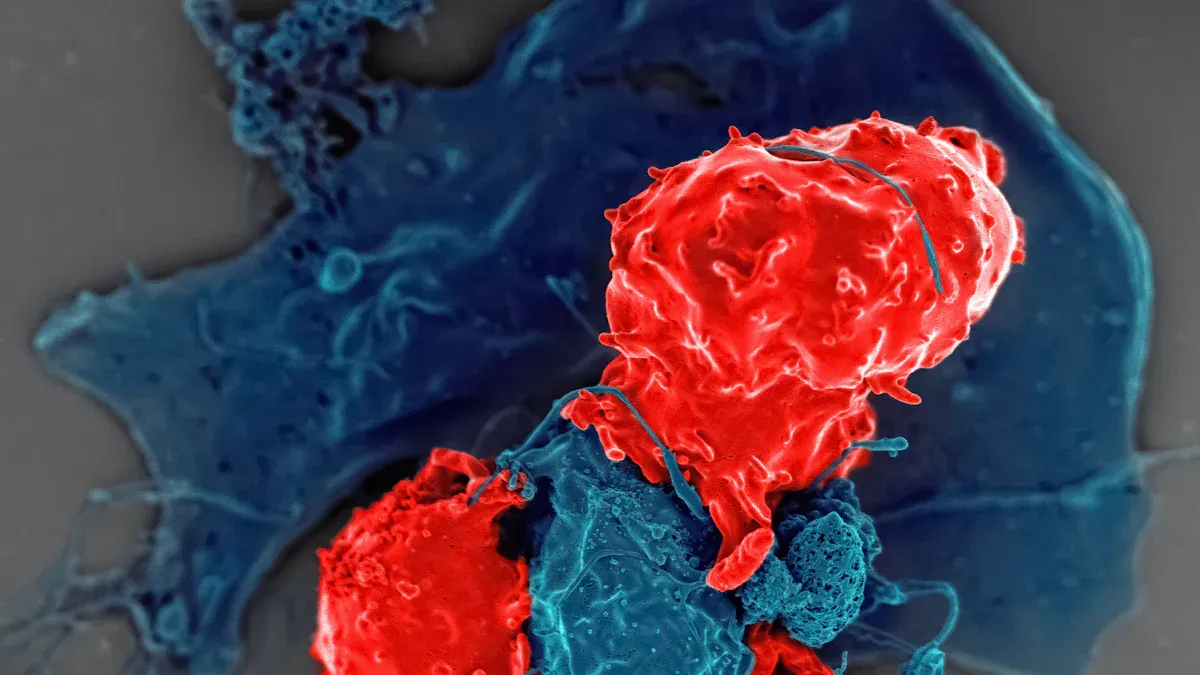
Immuno-oncology changes cancer treatment by boosting your body’s defenses. Traditional therapies like chemotherapy often bring harsh side effects and limited survival. The table below shows how immunotherapy improves patient outcomes and survival rates compared to older treatments:
| Treatment Type | Response Rates | Median Survival / Five-Year Survival Rates |
|---|---|---|
| Chemotherapy (Dacarbazine) | 10-20% | Less than one year |
| Immunotherapy (Checkpoint Inhibitors) | 30-40% | 30-40% (five-year) |
| Chemotherapy (NSCLC) | 20-30% | 10-12 months |
| Immunotherapy (Pembrolizumab) | 45% | 30 months (high PD-L1 expression) |
| Chemotherapy (Hodgkin Lymphoma) | 90% (early-stage) | 70-80% (five-year, advanced) |
| Immunotherapy (Nivolumab) | 69% | N/A |
You discover that immunotherapy includes passive and active approaches. Some therapies give your body ready-made immune tools. Others help your immune system create strong defenses, like special antibodies and T cells. These treatments target cancer cells directly, block tumor growth, and reduce side effects.
Key Takeaways
- Immuno-oncology uses your immune system to fight cancer, offering better outcomes and fewer side effects than traditional treatments.
- T cells are crucial in battling cancer. Immuno-oncology therapies boost T cell activity, helping them recognize and destroy cancer cells more effectively.
- Immune checkpoint inhibitors block signals that prevent T cells from attacking tumors, leading to improved survival rates in many cancers.
- New therapies like CAR T-cell therapy and cancer vaccines personalize treatment, enhancing your immune response against cancer.
- Ongoing research aims to improve immuno-oncology, focusing on better therapies and understanding how to predict patient responses.
Immuno-Oncology Overview
What Is Immuno Oncology
You may wonder how immuno oncology works. This field uses your immune system to find and destroy cancer cells. Unlike traditional treatments that attack cancer directly, immuno-oncology helps your body’s defenses do the work. Over the past decade, you have seen more than 150 FDA approvals for immunotherapy drugs. These therapies now treat over 30 types of cancer. Immuno-oncology includes several approaches that activate your immune system:
- Regulation of immune checkpoints to boost T-cell activity
- Oncolytic virus therapies that target and kill cancer cells
- Cancer vaccines that train your immune system to spot cancer-specific markers
- Cytokine therapies that strengthen immune responses
- Adoptive cell transfer, which gives you T-cells designed to fight cancer
How It Transforms Cancer Care
Immuno-oncology changes how you think about cancer treatment. You now have options that offer longer-lasting results and fewer side effects. The table below shows how immuno-oncology compares to traditional therapies:
| Characteristic | Immuno-Oncology | Traditional Therapies |
|---|---|---|
| Mechanism of Action | Engages the immune system | Directly attacks cancer cells |
| Durability of Response | More durable outcomes | Often less durable |
| Side Effect Profile | Less impact on quality of life | More severe side effects |
| Immune Memory | Creates long-term immune memory | No long-term immune memory |
| Adaptability | Can evolve to fight cancer | Static response |
Since 2011, the use of immunotherapy has grown more than 20 times. In 2024, new therapies like TIL and TCR-engineered treatments reached patients for the first time. You benefit from more targeted and personalized care.
Immune System and Cancers
Your immune system plays a key role in finding and destroying cancer. Sometimes, cancer cells hide or block your immune response. This allows tumors to grow. Immuno-oncology helps your immune system recognize and attack these hidden threats. Research shows that your immune system can control cancer growth by targeting tumor antigens. However, some cancers create an environment that weakens your immune response. Immuno-oncology strategies block these barriers and boost your body’s ability to fight back. You now have access to treatments that can help your immune system remember and attack cancer cells, even after many years.
Immune Activation in Cancer
T-Cell Activation
You play a key role in fighting cancer through your immune system. T cells act as your body’s soldiers. They find and destroy abnormal cells, including cancer cells. Immuno oncology uses this natural defense by boosting T cell activation. When you receive cancer immunotherapy, your T cells become more active and better at recognizing cancer.
Here is how T cell activation works during immuno-oncology therapy:
| Process | Description |
|---|---|
| Antigen Recognition | T cells recognize tumor-specific antigens presented by antigen-presenting cells (APCs) like dendritic cells. |
| Cytokine Release | Activated CD4+ T cells release IFN-γ, which stimulates other immune cells and has anti-tumor effects. |
| Metabolic Pathways | The tumor microenvironment can hinder T cell function. Understanding these pathways helps improve therapies. |
When you receive car t-cell therapy, your T cells are engineered to target cancer cells more accurately. These activated T cells multiply quickly, guided by cytokines such as interleukin-2. This rapid growth helps your immune system attack and kill cancer cells. Clinical trials show that car t-cell therapy leads to high response rates in patients with hard-to-treat cancers. The FDA approved CD-19 car t-cell therapy because it improves outcomes for people with relapsed or refractory cancers. You benefit from this activation, as it leads to the destruction of tumor cells and better survival rates.
Patient-reported outcomes show that T cell activation not only improves clinical results but also enhances your quality of life during treatment.
PD-1/PD-L1 Pathway
Cancer cells often use tricks to hide from your immune system. One major trick involves the PD-1/PD-L1 pathway. PD-1 is a protein on T cells. PD-L1 is a protein on tumor cells. When these two connect, your T cells become less active. This process helps tumors escape detection and grow.
Immuno-oncology therapies called immune checkpoint inhibitors block this pathway. By stopping PD-1 and PD-L1 from connecting, these drugs restore T cell activation. Your immune system can then attack cancer cells more effectively. Research shows that blocking the PD-1 pathway leads to stronger immune responses and better outcomes in many cancers. Clinical trials prove the value of checkpoint inhibition in improving survival.
| Study | Treatment | Overall Survival (OS) | Hazard Ratio (HR) | p-value |
|---|---|---|---|---|
| OAK (NCT02008227) | Atezolizumab | 13.8 months (ITT) | 0.73 | 0.0003 |
| OAK (NCT02008227) | Docetaxel | 9.6 months (ITT) | – | – |
| OAK (NCT02008227) | Atezolizumab | 15.7 months (PD-L1+) | 0.74 | 0.0102 |
| OAK (NCT02008227) | Docetaxel | 10.3 months (PD-L1+) | – | – |
| OAK (NCT02008227) | Atezolizumab | 12.6 months (TC0/IC0) | 0.75 | – |
| OAK (NCT02008227) | Docetaxel | 8.9 months (TC0/IC0) | – | – |
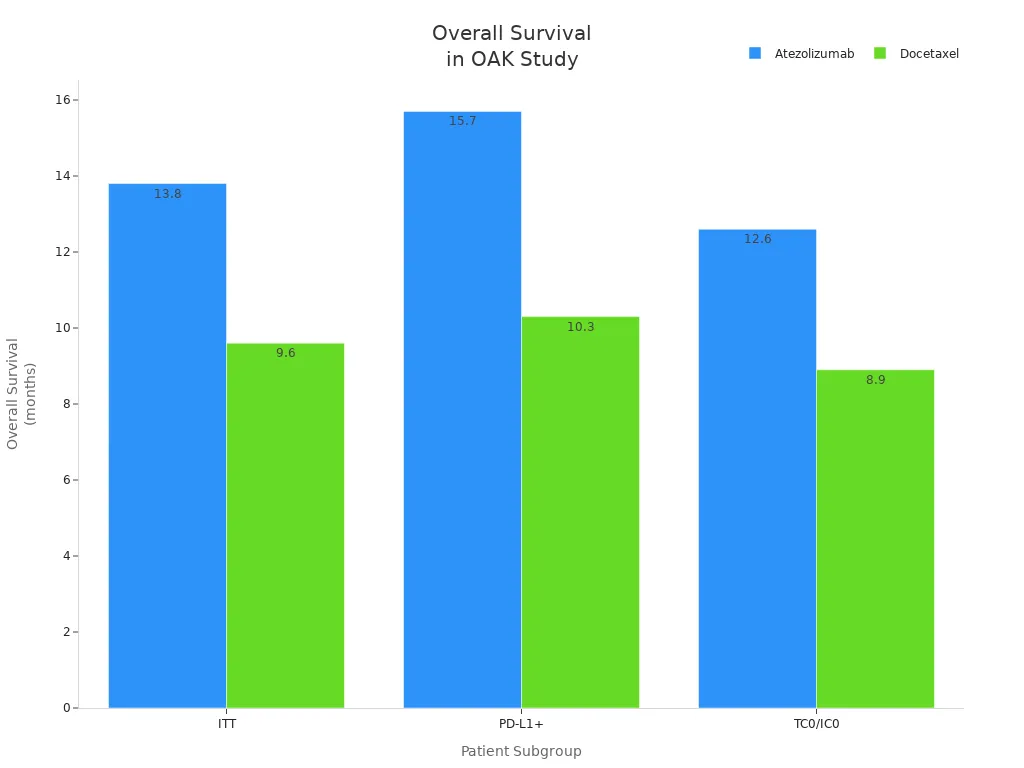
You see that patients treated with atezolizumab, an immune checkpoint inhibitor, live longer than those who receive standard chemotherapy. This success comes from disrupting the PD-1/PD-L1 axis and restoring tumor immunity.
Tumor Microenvironment
The tumor microenvironment shapes how well your immune system can fight cancer. This environment includes immune cells, blood vessels, fibroblasts, and the extracellular matrix. Sometimes, the tumor microenvironment blocks immune cells from reaching the tumor or weakens their attack.
| Mechanism | Description |
|---|---|
| Inhibitory Signals | The tumor microenvironment sends signals that help tumors grow and escape immune attack. |
| Physical Barriers | Structures like the extracellular matrix make it hard for immune cells to reach the tumor. |
| Metabolic Factors | The tumor microenvironment changes how immune cells use energy, which can weaken their response. |
| TME Component | Impact on Immunotherapy |
|---|---|
| Tumor-associated macrophages | These cells release cytokines that protect the tumor and increase drug resistance. |
| Tumor-associated fibroblasts | They help create an immunosuppressive environment, making treatment less effective. |
| Extracellular matrix | This acts as a barrier, stopping immune cells and drugs from reaching the tumor. |
A t-cell-inflamed tumor microenvironment helps your immune system work better. When you have more T cells inside the tumor, immunotherapy becomes more effective. However, some tumors create a non-inflamed environment, making it harder for your immune system to attack. Cancer cells can also recruit special cells that release substances like IL-10 and TGF-β. These substances suppress your immune response and help the tumor survive.
You can improve tumor immunity by targeting the tumor microenvironment. New immuno-oncology therapies focus on making the environment more favorable for T cell activation and checkpoint inhibition.
A t-cell-inflamed tumor microenvironment increases your chances of responding well to immunotherapy. By understanding and changing the tumor microenvironment, you help your immune system fight cancer more effectively.
Immuno-Oncology Therapies
Immuno oncology brings you a new era of cancer-fighting options. You now have access to therapies that use your immune system to find and destroy cancer cells. These immune therapies work in different ways, but they all focus on activation of your body’s natural defenses. Let’s explore the main types of immunotherapy and see how they improve cancer treatment and patient outcomes.
Immune Checkpoint Inhibitors
Checkpoint inhibitor immunotherapies have changed how you fight cancer. These drugs block proteins that usually stop your t cells from attacking tumors. When you use immune checkpoint inhibitors, you remove the “off” signals that cancer cells send to your immune system. This activation lets your immune system target and destroy cancer cells more effectively.
- PD-1 and PD-L1 inhibitors block the connection between PD-1 on t cells and PD-L1 on tumor cells. Pembrolizumab and Nivolumab are common examples.
- CTLA-4 inhibitors, like Ipilimumab, stop CTLA-4 from turning off your immune response.
- LAG-3 inhibitors, such as Relatlimab, boost immune activation by blocking another checkpoint.
When you receive checkpoint inhibitor immunotherapies, you keep your t cells active and ready to attack cancer cells.
Here’s how checkpoint inhibition works:
- Immune checkpoint proteins on t cells bind to partner proteins on tumor cells, sending an “off” signal.
- Immune checkpoint inhibitors block this binding, stopping the “off” signal.
- Your t cells stay active and attack cancer cells.
Checkpoint inhibitor immunotherapies show strong results in clinical trials for cancers like non-small cell lung cancer (NSCLC). You can see the benefits in the table below:
| Efficacy Measure | Hazard Ratio (HR) / Relative Risk (RR) | P-value |
|---|---|---|
| Progression-Free Survival | 0.838 | < 0.001 |
| Overall Survival | 0.747 | < 0.001 |
| Objective Response Rate | 1.311 | < 0.001 |
These numbers mean you have a better chance of living longer and seeing your tumor shrink when you use checkpoint inhibition. Combination immunotherapy with checkpoint inhibitors often leads to even better results, especially when paired with other anti-cancer treatments.
CAR T-Cell Therapy
Car t-cell therapy gives you a powerful, personalized cancer-fighting tool. Doctors collect your t cells and change them in the lab to recognize cancer cells. These engineered t cells, called CAR T-cells, go back into your body and attack cancer directly.
| Mechanism | Description |
|---|---|
| CAR T-cell Engineering | T cells are genetically modified to express chimeric antigen receptors (CARs) that recognize specific tumor antigens. |
| MHC-independent Killing | CAR T-cells can kill tumor cells without the need for major histocompatibility complex (MHC) presentation. |
| Pathways of Tumor Cell Death | CAR T-cells use perforin/granzyme and death receptor pathways to trigger cancer cell death. |
- Doctors collect t cells from your blood.
- They engineer these t cells to express CARs that target cancer cell antigens.
- After engineering, you receive the t cells back, and they multiply to attack cancer.
Car t-cell therapy works well for blood cancers. Clinical trials show high response rates:
| CAR-T products | Target | Year | Clinical trial | Indications | Overall Response Rate (ORR) | Complete Response (CR) | Toxicities (Grade 3/4) |
|---|---|---|---|---|---|---|---|
| tisagenlecleucel | CD19 | 2017 | ELIANA | R/R B-ALL | 81% | 60% | CRS (46%), CRES (13%) |
| axicabtagene ciloleucel | CD19 | 2017 | ZUMA-1 | R/R DLBCL | 82% | 54% | CRS (13%), CRES (28%) |
| brexucabtagene autoleucel | CD19 | 2020 | ZUMA-2 | R/R MCL | 85% | 59% | CRS (15%), CRES (31%) |
| idecabtagene vicleucel | BCMA | 2021 | KarMMa | R/R MM | 73% | 33% | CRS (5%), CRES (3%) |
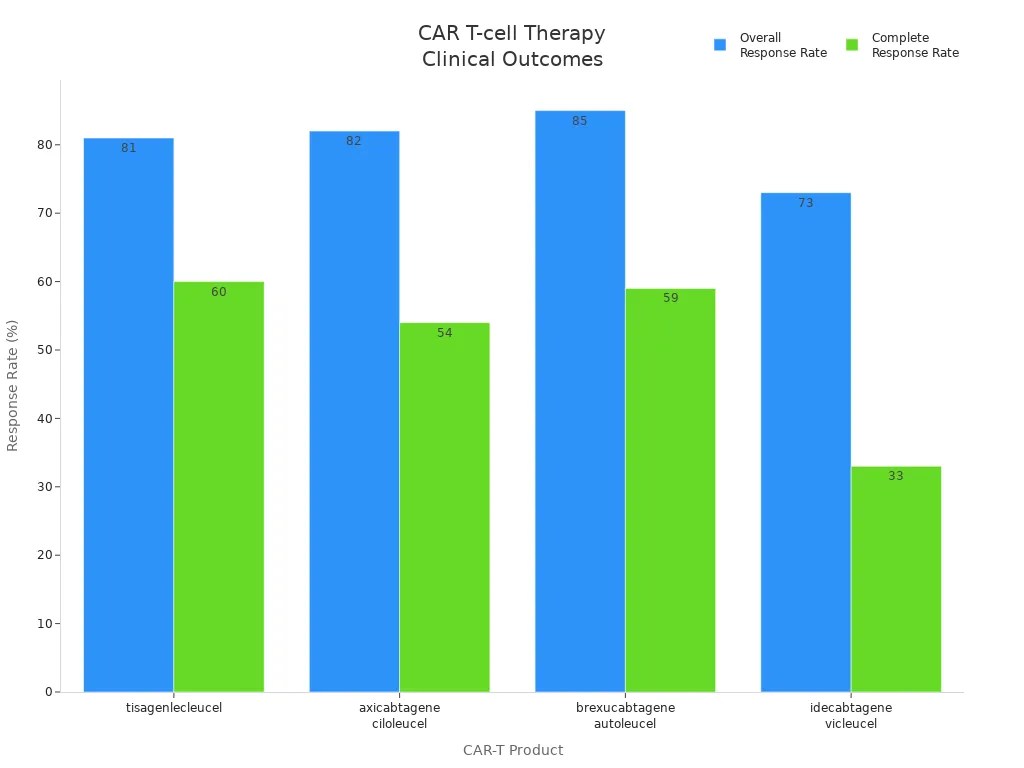
You see that car t-cell therapy leads to high rates of remission, even in hard-to-treat cancers. However, you should know that side effects like cytokine release syndrome (CRS) and neurotoxicity can occur. Doctors monitor you closely to manage these risks.
Combination immunotherapy with car t-cell therapy and other treatments is under study. These combinations may improve tumor immunity and help more patients.
Cancer Vaccines
Cancer vaccines help your immune system recognize and attack cancer cells. You can receive different types of vaccines:
- Preventive cancer vaccines protect you from viruses that cause cancer, like HPV and HBV.
- Therapeutic cancer vaccines target proteins found on your tumor, such as the sipuleucel-T vaccine for prostate cancer.
- Personalized neoantigen vaccines use unique mutations in your tumor to create a custom immune response.
A recent review of clinical trials shows that cancer vaccines can help you live longer, especially if you have advanced non-small cell lung cancer. Patients with squamous cell carcinoma see the most benefit. Your immune response to the vaccine may predict how well you do with this treatment.
Combination immunotherapy with cancer vaccines and checkpoint inhibitors is a growing area. These combinations may boost activation of your immune system and improve cancer-fighting results.
Oncolytic Viruses
Oncolytic viruses are a special kind of immunotherapy. These viruses infect and kill cancer cells, but leave healthy cells alone. When the virus enters a tumor cell, it multiplies and causes the cell to burst. This releases tumor antigens and triggers a strong immune response.
- Oncolytic viruses activate both innate and adaptive immune responses.
- They bind to cancer cell receptors, enter the cells, and cause cell lysis.
- Dying cancer cells release signals that attract immune cells and boost t-cell activation.
Oncolytic virotherapy not only kills cancer cells directly but also helps your immune system recognize and attack more tumor cells.
You may receive oncolytic viruses alone or as part of combination immunotherapy. When combined with checkpoint inhibition or chemotherapy, you often see better disease control. Doctors watch for side effects like fever and fatigue, but most patients tolerate this treatment well.
Targeted Antibodies
Targeted antibodies are proteins that find and attach to specific markers on cancer cells. Once attached, they disrupt cancer cell growth and signal your immune system to destroy the tumor. Most targeted antibodies are passive immunotherapies, but some new types also activate immune cells.
- Targeted antibodies bind to cancer cell antigens and block growth pathways.
- They alert other immune cells to attack the tumor.
- Antibody-drug conjugates (ADCs) deliver toxic drugs straight to cancer cells, reducing side effects.
- Bispecific antibodies can link t cells to cancer cells, boosting immune response.
Combination immunotherapy with targeted antibodies and other treatments, like checkpoint inhibitors or chemotherapy, shows strong results. The table below highlights key advances:
| Combination Therapy | Cancer Types | Key Findings |
|---|---|---|
| ADCs + Immune Checkpoint Inhibitors | HER2-positive breast cancer, triple-negative breast cancer, urothelial carcinoma | Increases progression-free survival and tumor-specific responses. |
| ADCs + Chemotherapy | Non-small cell lung cancer, breast cancer, lymphoma | Enhanced tumor response and synergistic cytotoxic effects. |
| ADCs + Targeted Small-Molecule Inhibitors | Lung cancer, breast cancer, other solid tumors | Improves outcomes in cancers resistant to monotherapy. |
| ADCs + Anti-Angiogenic Agents | HER2-positive breast cancer, ovarian cancer | Increases ADC penetration in tumors and improves progression-free survival. |
| ADCs + CAR-T Cell Therapy | Hematological malignancies, solid tumors | Synergistic effects observed in hematologic cancers like leukemia and lymphoma. |
| ADCs + Immune Modulators | Melanoma, breast cancer, ovarian cancer | Enhances immune response and reduces tumor growth. |
| ADCs + Other Immunotherapies | Melanoma, non-small cell lung cancer, head and neck cancers | Increases T-cell activation and tumor rejection, showing promising results in early-phase trials. |
You benefit most when doctors use combination immunotherapy strategies. These combinations increase activation of your immune system, improve tumor immunity, and help you fight cancer more effectively. Immuno-oncology continues to bring new hope and better outcomes for people facing cancer.
Challenges and Future of Immuno Oncology
Patient Response
You may notice that not every person responds the same way to immuno-oncology therapies. Many factors shape how your body fights cancer:
- You may need more biomarkers to predict who will benefit.
- Tumor heterogeneity means each cancer can look and act differently.
- Your past treatments can change how your immune system reacts.
- Some cancers have strong immunosuppressive biology.
- The gut microbiota, which includes many microorganisms, can affect how well your immune system works.
- Your immune system’s strength and diversity matter.
Differences in immune system competency and diversity can lead to different response rates. Chemotherapy may lower your immune system’s ability to fight, which affects how well you respond.
Researchers have found that greater diversity in HLA class I molecules links to better survival after immuno-oncology therapy. This diversity may help predict how well you respond to cancer treatment.
Side Effects
Immuno-oncology can cause side effects. You should know how to manage them. Here is a table showing common side effects and ways to handle them:
| Side Effect | Management Strategy |
|---|---|
| Gastrointestinal Issues | Monitor bowel movements, avoid certain foods, hydrate, consult your provider. |
| Flu-Like Symptoms | Rest, drink fluids, consult your provider if symptoms worsen. |
| Respiratory Problems | Seek medical advice for ongoing symptoms. |
| Endocrine Disorders | Regular checks, possible hormone therapy. |
| Fatigue | Take short naps, do light exercise, stay hydrated. |
| Diarrhea and Immune Colitis | Avoid irritating foods, hydrate, monitor symptoms. |
| Mucositis | Use alcohol-free mouthwash, keep lips moist, consult your provider. |
| Neuropathy | Exercise, avoid alcohol, consider acupuncture. |
| Skin Changes | Moisturize skin, avoid sunlight, consult your provider. |
Ongoing Research
Ongoing research continues to improve how you fight cancer. Scientists focus on several areas:
| Research Area | Description |
|---|---|
| Checkpoint Inhibitory Therapy | Studies how cancer cells avoid your immune system and how to block these tricks. |
| CAR T Cell Therapy | Engineers your T cells to target and destroy cancer cells more effectively. |
| Cancer Vaccines | Helps your immune system recognize new cancer markers and attack tumors. |
You benefit from these advances as researchers discover new ways to boost your immune response.
Future Directions
You can expect even more progress in the future. Here are some trends shaping cancer care:
- Immuno-oncology now helps more people achieve long-term survival.
- Some therapies cure cancer when used before or after surgery.
- Cell therapies, like CAR T and TCR, are becoming easier to use.
- Scientists invest in basic biology and smarter biomarker strategies.
- AI tools help find better drug targets and improve trial design.
- Intratumoral delivery lets doctors treat cancer locally.
- AI-driven biomarkers may soon predict who will benefit most.
- Expanding access and equity ensures more people get these new treatments.
Recent studies show that immuno-oncology improves survival rates for many cancers. Some patients now live years longer, and a few reach survival rates close to the general population. Ongoing research and new therapies give you hope for better outcomes in the fight against cancer.
Immuno-oncology gives you new hope in the fight against cancer. You see how activating your immune system leads to better outcomes and fewer side effects. Staying informed about new therapies helps you make smart choices.
Keep learning about advances in cancer care. You play a key role in your health journey. The future looks brighter as science brings more effective and personalized treatments.
FAQ
What is the main goal of immuno-oncology?
You use immuno-oncology to help your immune system find and destroy cancer cells. This approach aims to give you longer-lasting results and fewer side effects than traditional cancer treatments.
Are immuno-oncology therapies safe for everyone?
Most people tolerate these therapies well. Some patients may experience side effects like fatigue or skin changes. Your doctor will monitor you closely and adjust your treatment if needed.
How do immune checkpoint inhibitors work?
Immune checkpoint inhibitors block signals that stop your T cells from attacking cancer. You keep your immune system active, which helps you fight cancer more effectively.
Can immuno-oncology cure cancer?
Some patients achieve long-term remission or even a cure, especially with early treatment. Results depend on your cancer type, stage, and how your immune system responds.