News & Events
Can Monoclonal Antibody Polyclonal Antibody Stop Rejection Fast?
Organ transplant patients face a real risk of antibody-mediated rejection, especially in pediatric groups, where rates can reach up to 40%.
| Age Group | Cumulative Incidence of ABMR |
|---|---|
| Pediatric | Up to 40% |
| Adult | Varies |
Transplant specialists often rely on monoclonal antibody polyclonal antibody therapies to stop rejection quickly. Studies show polyclonal antibodies usually control rejection faster and more effectively than monoclonal options. Rapid control matters because it helps more than 80% of grafts survive and function well in the first year after treatment.
Key Takeaways
- Monoclonal and polyclonal antibodies are crucial in preventing organ transplant rejection. They help protect the transplanted organ from the immune system’s attack.
- Polyclonal antibodies often work faster and more effectively than monoclonal antibodies, especially in severe cases of rejection. This can lead to better survival rates for the transplanted organ.
- Doctors choose antibody therapies based on the patient’s risk of rejection and the type of transplant. Monoclonal antibodies are preferred for less severe cases, while polyclonal antibodies are used for high-risk patients.
- Both types of antibodies have side effects. Monoclonal antibodies generally have a safer profile, while polyclonal antibodies can increase the risk of infections.
- Patients should discuss their specific needs with transplant specialists to find the best antibody therapy for their situation. Tailored treatment can improve outcomes and protect the transplanted organ.
Monoclonal Antibody in Transplantation
Mechanism
Monoclonal antibody polyclonal antibody therapies play a key role in transplantation. Monoclonal antibodies work by targeting specific cells or proteins in the immune system. In transplantation, these antibodies often focus on B cells, which produce harmful antibodies that attack the transplanted organ. The table below shows how monoclonal antibodies act on different parts of the immune system:
| Mechanism/Aspect | Description |
|---|---|
| Role of B cells | B cells and anti-HLA antibodies are crucial in acute and chronic allograft rejection. |
| CD20+ B cells | Presence correlates with irreversible acute rejection episodes. |
| C4d deposition | Sensitive and specific marker for acute antibody-mediated rejection. |
| Rituximab | Effective in some acute humoral rejection cases by eliminating CD20-expressing B cells. |
| Limitations of Rituximab | Does not affect plasma cells and ectopic lymphoid structures in chronic inflammation. |
Monoclonal antibody polyclonal antibody treatments like rituximab target CD20+ B cells. This action helps stop the production of harmful antibodies during transplantation.
Speed of Action
Doctors often choose monoclonal antibody polyclonal antibody therapies for their targeted and sometimes rapid effects. Studies show that adding rituximab to standard treatments can improve graft survival rates. The table below compares different treatment protocols:
| Study | Treatment Protocol | Graft Survival | Rejection Rate |
|---|---|---|---|
| Vo et al | IVIG + Rituximab | 94% | 50% |
| Lefaucheur et al | PLEX + IVIG + Rituximab | 91.7% | N/A |
| Lefaucheur et al | IVIG alone | 50% | N/A |
These results show that monoclonal antibody polyclonal antibody therapies can act quickly and improve outcomes in transplantation.
Effectiveness
Monoclonal antibody polyclonal antibody therapies have shown strong results in clinical trials. For example, a study using OKT3 monoclonal antibody in kidney transplantation found a 94% reversal rate of rejection, much higher than the 75% rate with steroids. The one-year graft survival rate was also better with OKT3. Other studies support these findings. The chart below shows graft survival rates from several studies using monoclonal antibody polyclonal antibody therapy for acute rejection:
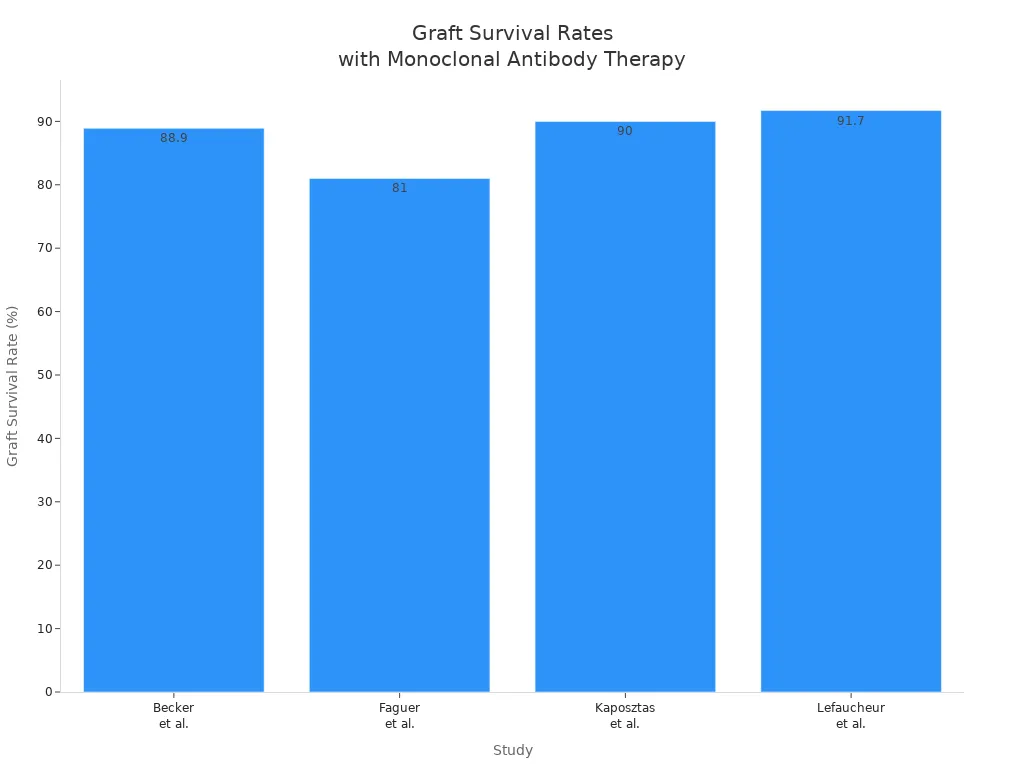
These results highlight the effectiveness of monoclonal antibody polyclonal antibody treatments in transplantation, especially for stopping antibody-mediated rejection.
Polyclonal Antibody in Transplantation
Mechanism
Polyclonal antibodies provide broad immune modulation in transplantation. These antibodies come from multiple immune cells, so they can target many different parts of the immune system. This wide action helps control both T cells and B cells, which play major roles in transplant rejection. Researchers have studied how polyclonal antibodies affect immune responses in transplantation. The table below shows some findings:
| Evidence Description | Findings | Implications |
|---|---|---|
| LIGHT-blocking polyclonal antibody | Partially inhibited the course of GvHD | Suggests potential for polyclonal antibodies in reducing GvHD severity |
| Administration of soluble LTβR-Ig | Attenuated rejection of host hematopoietic cells | Indicates a role in modulating CTL responses during transplantation |
Polyclonal antibodies can also reduce the activity of alloreactive T cells. In a phase 1 study, Fueyo and colleagues found that these therapies lowered T cell activity by over 20%. This supports their use in liver transplantation and other settings.
Onset and Duration
Polyclonal antibodies act quickly after administration. Doctors often use them when rapid immune suppression is needed in transplantation. The onset of action usually occurs within hours to a few days. The effects can last for several weeks, depending on the dose and type of antibody used. This fast and lasting action makes polyclonal antibodies valuable for both preventing and treating rejection episodes.
Clinical Use
Clinicians use polyclonal antibodies in several ways during transplantation. They help prevent rejection in high-risk patients and treat antibody-mediated rejection when it occurs. Common therapies include intravenous immunoglobulin (IVIG) and immunoadsorption. These treatments work by neutralizing harmful antibodies and blocking complement activity.
- IVIG is often combined with plasmapheresis to manage antibody-mediated rejection.
- Immunoadsorption removes harmful antibodies and immune complexes, especially in highly sensitized patients. In a study by Qing and colleagues, this approach reduced both class I and II panel-reactive antibodies and complement levels.
Polyclonal antibodies remain a key tool in transplantation because they offer broad and fast immune control.
Monoclonal Antibody Polyclonal Antibody: Comparison
Speed and Efficacy
Doctors often choose between monoclonal and polyclonal antibodies based on how quickly they need to control transplant rejection. Monoclonal antibodies, such as basiliximab and rituximab, target specific immune cells. This targeted action can stop the immune attack on a transplanted organ quickly, especially in cases of acute rejection. Studies show that basiliximab induction leads to a lower rate of acute rejection and a shorter hospital stay for patients after solid organ transplantation.
| Group | Acute Rejection Rate | Length of Hospital Stay | P-Value |
|---|---|---|---|
| Basiliximab Induction | Lower incidence | Reduced | 0.002 |
| No Induction | Higher incidence | Increased | N/A |
Polyclonal antibodies, such as anti-thymocyte globulin (ATG), provide broad immune suppression. They act on many types of immune cells, which can make them more effective for severe or resistant transplant rejection. In high-risk patients, polyclonal antibodies show a greater reduction in rejection rates compared to monoclonal antibodies. For example, in kidney transplant recipients, ATG reduced the risk of acute rejection more than IL2RA (a monoclonal antibody), especially in patients with a high risk of rejection.
| Comparison | Outcome | Risk Reduction | KTR Group |
|---|---|---|---|
| ATG vs IL2RA | Acute rejection | RR 0.74 (95% CI 0.61–0.89) | Standard KTR |
| ATG vs IL2RA | Acute rejection | RR 0.55 (95% CI 0.43–0.72) | High risk of rejection KTR |
Both types of antibodies can stop transplant rejection fast, but polyclonal antibodies often work better for severe or resistant cases. Monoclonal antibodies may be preferred for their quick action and shorter hospital stays in less severe cases of solid organ transplantation.
Safety
Safety is a key concern when treating transplant rejection. Monoclonal antibodies have a more targeted effect, which lowers the risk of infections after solid organ transplantation. For example, basiliximab does not cause lymphocyte depletion, so patients have fewer infections compared to those treated with polyclonal antibodies. This targeted approach also means fewer non-specific side effects and better overall survival for both the patient and the transplanted organ.
Polyclonal antibodies, while effective, can cause more side effects. They suppress the immune system broadly, which increases the risk of infections and other complications. The table below lists some of the most common adverse effects for both therapies:
| Adverse Effect | Description |
|---|---|
| Infectious complications | Increased risk due to loss of innate immune response from immunosuppressive agents. |
| Malignancies | Rare in anti-CD25 treated patients; less frequent compared to ATG-treated patients. |
| Myelosuppression | Leukopenia (10%-15%) and thrombocytopenia (5%) reported in basiliximab-treated patients. |
| Eculizumab Side Effects | Common adverse events include hypertension, diarrhea, headache, and severe Neisseria infections. |
Doctors must weigh these risks when choosing a therapy for transplant rejection. Monoclonal antibodies offer a safer profile for many patients, while polyclonal antibodies may be necessary for those with severe or resistant rejection.
Clinical Scenarios
The choice between monoclonal and polyclonal antibodies depends on the patient’s risk factors, the type of solid organ transplantation, and the severity of transplant rejection. The table below summarizes how these factors influence therapy choice:
| Therapy Type | Rejection Rate (%) | Infection Rate | Notes |
|---|---|---|---|
| Polyclonal Antibodies | Lower | Higher | Strong immunosuppression, increased infection risk. |
| Monoclonal Antibodies | Higher | Lower | Profound initial lymphopenia, shorter time to rejection onset. |
| General Findings | N/A | N/A | Some studies show no significant difference in rejection rates, indicating variability. |
Doctors often use polyclonal antibodies for patients at high risk of transplant rejection or those who have already failed other treatments. These patients may need stronger immune suppression, even if it means a higher risk of infection. Monoclonal antibodies are often chosen for patients with a lower risk of rejection or those who need a safer option after solid organ transplantation.
Cost-effectiveness also plays a role. Studies show that depletional therapies like r-ATG (a polyclonal antibody) offer a good balance of cost and benefit over three years. r-ATG is more effective and provides better outcomes for high-risk patients, even though it costs more than some monoclonal options.
| Induction Type | Cost Advantage | Outcome Advantage | Time Frame |
|---|---|---|---|
| Depletional (r-ATG) | Substantial | Most beneficial balance | 3 years |
Doctors must consider each patient’s needs, the type of solid organ transplantation, and the risk of transplant rejection when choosing the best therapy. Both monoclonal and polyclonal antibodies have important roles in modern transplant medicine.
Antibody-Mediated Rejection: Practical Considerations
Choosing the Right Antibody
Doctors must consider many factors when selecting antibody therapy for antibody-mediated rejection. The cost of treatment can be high, especially for patients without insurance. Even with generic drugs, many patients face large out-of-pocket expenses for long-term immunosuppressive therapy. Medicare coverage often ends three years after transplant, which can lead to non-adherence and increase the risk of rejection. Availability of certain therapies may also vary by hospital or region. Doctors must balance these financial and access issues with the need to control rejection quickly and protect the donor organ.
Patient Factors
Each patient brings unique challenges to antibody therapy. The timing and number of rejection episodes affect long-term outcomes. Patients who have had previous rejection episodes are more likely to develop donor-specific antibodies, which can threaten the donor organ. Comorbidities, such as diabetes or heart disease, can change how well immunosuppressive therapies work. The nature of rejection—whether it is acute or antibody-mediated—also shapes the response to treatment and the chance of keeping the donor organ healthy.
| Patient-Specific Factor | Impact on Therapy Outcomes |
|---|---|
| Previous Rejection Episodes | Higher risk of developing donor-specific antibodies and possible graft loss. |
| Comorbidities | Can reduce the effectiveness of immunosuppressive therapies and affect donor outcomes. |
| Nature of Rejection | Acute vs. antibody-mediated rejection changes therapy response and long-term donor health. |
Doctors also look at age and medical history. Younger age at heart transplant and congenital heart disease can increase the risk of losing the donor organ after antibody-mediated rejection. Rejection with hemodynamic compromise can make outcomes worse.
Limitations
Both monoclonal and polyclonal antibody therapies have important limitations. Long-term success rates remain unclear, and patients face a higher risk of severe infections. Doctors find it hard to compare treatments because studies use different definitions and methods. Ethical concerns make it difficult to run placebo-controlled trials, as untreated rejection can lead to donor organ loss. Small study sizes and inconsistent results add to the challenge.
Side effects are another concern. Patients may experience infusion reactions, skin problems, high blood pressure, or stomach issues. Some may have diarrhea, fever, cough, or even hormone problems like hypothyroidism.
Note: Doctors must weigh the benefits and risks of each therapy. They need to consider the patient’s history, the type of rejection, and the risk to the donor organ. No single therapy works for every patient, so treatment must be tailored to each case.
Polyclonal antibodies often stop rejection faster in liver transplantation, especially when severe inflammation threatens graft survival. Monoclonal antibodies, like IL2Ra, remain first-line for many patients. Both types show similar five-year survival rates in liver transplant recipients, with no major differences in long-term transplant outcomes. Recent advances, such as felzartamab and belimumab, offer new hope for reducing inflammation and improving survival after liver transplantation. Clinicians should match antibody therapy to each patient’s needs, always aiming to protect liver function and long-term survival. Patients should talk with transplant specialists to choose the best treatment for liver transplantation.
FAQ
What are antibodies and why do they matter in transplant rejection?
Antibodies are proteins that help the immune system find and attack foreign substances. After a transplant, antibodies can target the new organ. This attack can harm the graft and lower graft survival. Doctors watch for antibodies to protect the graft.
How do monoclonal and polyclonal antibodies work in transplant patients?
Monoclonal antibodies target one specific part of the immune system, like b-cells or hla proteins. Polyclonal antibodies act on many immune cells. Both types help stop humoral rejection and protect the graft from immune responses.
Why do doctors monitor hla and igg levels after a transplant?
Doctors check hla and igg levels to see if the immune system is making antibodies against the graft. High levels can mean the body is attacking the graft. Early detection helps doctors act fast to save graft survival.
Can antibodies from b-cells cause humoral rejection in all types of transplants?
Yes, b-cells can make antibodies that cause humoral rejection in kidney, liver, and heart transplants. These antibodies often target hla proteins. Fast treatment helps protect the graft and improve graft survival.
What is the role of igg in antibody-mediated rejection?
Igg is a type of antibody that can bind to the graft and start immune responses. High igg levels can lead to humoral rejection. Doctors use therapies to lower igg and protect the graft after a transplant.