News & Events
Advances in Protein Production Techniques in 2025

You see major advances in protein production techniques in 2025. Proteins are made where in the cell, and scientists now use new strategies to boost efficiency and quality. Proteins come from engineered Bacillus subtilis, improved plant-based systems, and optimized recombinant methods. Proteins show better yields with metabolic engineering, promoter refinement, and secretion pathway changes. Proteins from lettuce plants offer scalable and safe solutions. Proteins made in E. coli benefit from enhanced glycosylation and disulfide bond formation. Proteins drive market growth with a projected CAGR of 6.7%. Proteins fuel biopharmaceutical R&D and personalized medicine. Proteins produced in yeast and mammalian cells deliver new therapies. Proteins are easier to purify with advanced technologies. Proteins reach higher purity levels with modern equipment. Proteins support researchers, students, and biotech professionals. Proteins improve vaccine and therapeutic production. Proteins enable better troubleshooting and optimization. Proteins create opportunities for sustainable synthesis.
Tip: You can choose plant-based systems or engineered bacteria to increase protein yield and purity.
| Key Development | Description |
|---|---|
| Engineering Bacillus subtilis | Focus on metabolic engineering to enhance protein production capabilities. |
| Plant-based systems | Lettuce-based platforms offer scalable and robust protein production. |
Key Takeaways
- New protein production techniques in 2025 enhance efficiency and quality, leading to better yields.
- Choosing the right expression system, like E. coli or plant-based systems, can significantly improve protein yield and purity.
- Automation and machine learning streamline protein synthesis, reducing errors and speeding up research.
- Using maltose-binding protein (MBP) as a fusion partner increases solubility and simplifies purification processes.
- Sustainable protein production methods, such as precision fermentation and cellular agriculture, are crucial for reducing environmental impact.
Proteins Are Made Where in the Cell

Ribosomes and Protein Synthesis
You often ask, “proteins are made where in the cell?” The answer centers on ribosomes. Ribosomes act as the main sites for protein synthesis. You find ribosomes floating freely in the cytoplasm or attached to the endoplasmic reticulum. When you look at immature red blood cells, you see ribosomes working hard to make hemoglobin. Ribosomes join amino acids together, forming chains that fold into functional proteins. The endoplasmic reticulum helps ribosomes make secreted and membrane-bound proteins. You rely on ribosome profiling technologies, like Ribo-seq, to study how ribosomes control protein synthesis. These tools give you a clear view of how ribosomes work in different cells. Recent advances in cryo-electron tomography let you watch ribosome behavior inside living cells. You discover that some ribosomes have special structures and functions. This knowledge helps you improve protein production techniques.
Note: Ribosomes are essential for protein synthesis. You need them to assemble amino acids and create proteins with the right structure and function.
| Aspect | Prokaryotes | Eukaryotes |
|---|---|---|
| Ribosomal Structure | 70S ribosomes (30S + 50S subunits) | 80S ribosomes (40S + 60S subunits) |
| Location | Cytoplasm | Cytoplasm and endoplasmic reticulum |
Transcription and Translation
You start with transcription. In this process, you use DNA as a template to make mrna. Transcription happens in the nucleus of eukaryotic cells. You copy the genetic code from DNA to mrna. After transcription, you move to translation. Translation uses mrna as instructions. Ribosomes read mrna and build proteins by linking amino acids. In prokaryotic cells, transcription and translation happen at the same time in the cytoplasm. In eukaryotic cells, transcription occurs in the nucleus, and translation takes place in the cytoplasm. You notice that mrna in eukaryotes gets a 5′ cap and a poly-A tail for stability. Genes in eukaryotes have introns and exons, so you need to splice mrna before translation. You pay attention to translation regulation. If elongation or termination goes wrong, you get misfolded or non-functional proteins. This can cause cellular problems. Quality control mechanisms help you ensure that proteins fold and function correctly.
- Elongation deregulation can change protein abundance and quality.
- Termination issues can create non-functional proteins.
- Deregulation of translation can lead to protein misfolding and disease.
| Aspect | Insight |
|---|---|
| Translation Regulation | You must regulate translation initiation, elongation, and termination for good protein yield and quality. |
| Quality Control Mechanisms | You rely on these to ensure correct folding and targeting of proteins. |
| Implications of Deregulation | Problems in translation can cause misfolding and cellular dysfunction. |
You see that understanding transcription and translation helps you boost protein synthesis. You improve yield and quality by controlling these steps. When you ask, “proteins are made where in the cell,” you learn that ribosomes, mrna, and the endoplasmic reticulum all play key roles.
Protein Expression Systems
You have many choices for protein expression. Each system offers unique benefits for yield, cost, and complexity. You can select prokaryotic, eukaryotic, or cell-free systems based on your needs.
Prokaryotic Expression
You often use E. coli for protein expression. This system grows fast and costs less. You can produce several grams of protein per liter. E. coli works well for simple proteins, but it cannot add complex modifications. You may choose E. coli when you want high yield and easy scale-up. You see that yeast systems, like Pichia pastoris, can perform post-translational modifications. Yeast gives you high-level expression and secretion. You use yeast for proteins that need glycosylation.
| Expression System | Yield (g/L) | Post-Translational Modifications | Notes |
|---|---|---|---|
| E. coli | Up to several grams | No | Rapid growth, cost-effective, but limited in complexity. |
| Pichia pastoris | Up to 10 | Yes | High-level expression and secretion capabilities. |
Eukaryotic Expression
You use insect cells and mammalian cells for more complex proteins. Insect cells, like Sf9, help you express larger membrane proteins. You get yields from 0.1 to over 1 g/L. Mammalian cells, such as CHO cells, produce proteins with human-like modifications. You rely on mammalian systems for therapeutic proteins. You see that plant systems offer scalable and cost-effective solutions, but you still work to improve their yields.
| Expression System | Cost | Scalability | Post-Translational Modifications |
|---|---|---|---|
| E. coli | Low | High | Limited (no complex glycosylation) |
| Yeast | Moderate | Moderate | N- and O-glycosylation (hypermannose) |
| Insect | Moderate | High | Limited glycosylation (paucimannose) |
| Mammalian | High | Moderate | Complex N-glycosylation (human-like) |
You can compare the maximum protein yields visually:
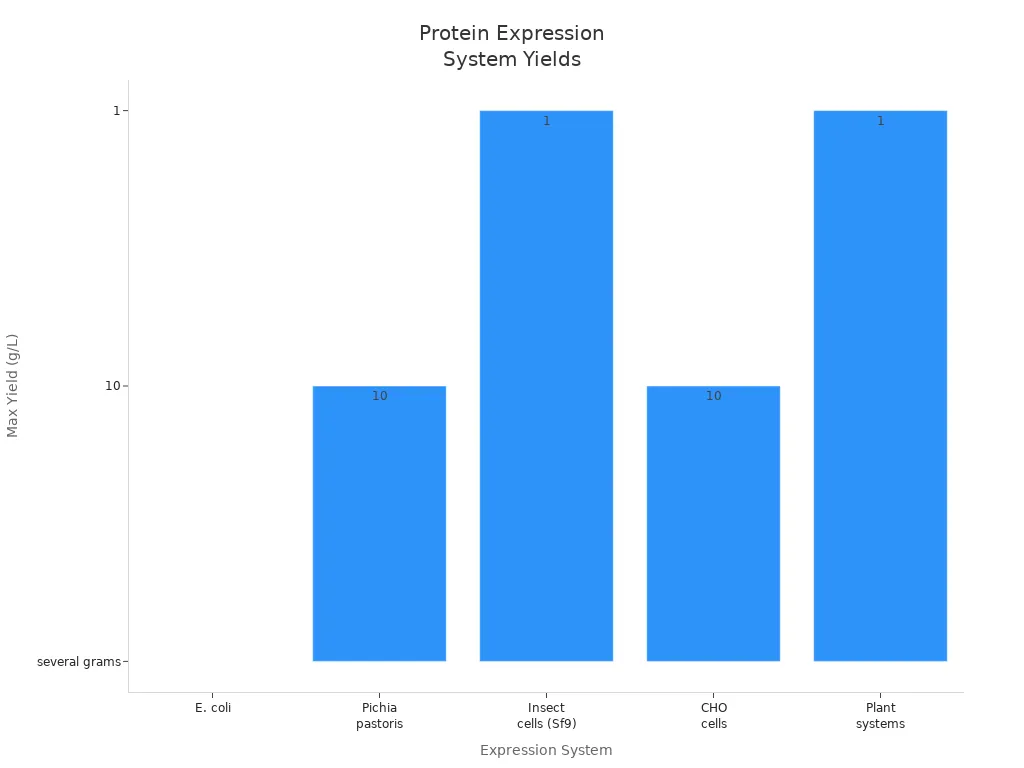
Tip: You should choose your system based on the complexity of your protein and your budget.
Cell-Free Synthesis
You can use cell-free protein synthesis systems for rapid protein production. These systems let you skip cell growth and cloning. You can optimize protein expression easily. You produce proteins from synthetic DNA in hours. You avoid cell toxicity issues and express proteins that are hard to make in living cells. NEBExpress and PUREfrex systems give you flexible reaction conditions and minimal protease activity. You get quick results and stable proteins. You may face challenges from nonspecific nucleases and proteases in extract-based systems. You see that cell-free systems reduce time and cost for difficult proteins. You can customize conditions for maximum yield.
- You optimize protein expression quickly.
- You produce proteins from synthetic DNA.
- You express toxic proteins without cell sensitivity.
- You save costs by reducing purification steps.
- You get analytical amounts of protein fast.
- You enjoy flexible reaction conditions.
- You may encounter nonspecific nucleases and proteases.
You find that cell-free protein synthesis systems are expanding into therapeutic protein production and synthetic biology. You expect future systems to deliver even higher yields and better protein folding.
Advances in Synthesis Technology
Automation and High-Throughput
You see automation changing the way you approach protein synthesis. Automated systems now handle many steps that once required careful manual work. Liquid-handling robots can pipette tiny volumes, mix solutions, and control temperatures with high precision. These robots help you achieve high-level protein production and reduce human error. You can run many synthesis reactions at once, which speeds up your research and increases your yield.
Automation systems also help you translate complex protocols into reliable, repeatable steps. You no longer worry about losing scientific validity when you automate. Careful design ensures that each step in the synthesis process remains accurate. You benefit from high-level expression and consistent results.
| Technology Type | Description | Benefits |
|---|---|---|
| Liquid-handling Robots | Enable increased throughput with less human labor and reduced error. | High-throughput protein production and purification, though expensive and requiring training. |
| Automation Systems | Facilitate the automation of tedious and error-prone steps in protein synthesis. | Expedited protein research with high yields and purity. |
You must pay attention to details like pipetting accuracy and temperature control. Automated systems can handle volumes from 1 μL to 5 mL and keep temperatures steady from 4°C to 37°C. These features are essential for successful synthesis and high-level expression. You also rely on mechanical movements, such as mixing and centrifugation, to complete multistep protocols.
| Challenge | Description | Solution |
|---|---|---|
| Protocol Translation | Translating multistep protocols into automation without losing validity. | Requires careful design to maintain scientific integrity while automating processes. |
| Operational Intricacies | Need for accurate pipetting and temperature control. | Automation systems must be designed to handle these intricacies effectively. |
Tip: Automation lets you scale up synthesis and purification, making high-level protein production more accessible.
Machine Learning in Protein Expression
You use machine learning to optimize protein synthesis and expression. These models help you predict which DNA sequences will give you the best results. You do not need as many training sequences as before. Machine learning now achieves high prediction accuracy with fewer examples. This means you can reach high-level expression faster and with less trial and error.
You see that controlled sequence diversity improves data efficiency. You get better model performance even with smaller datasets. This shift in approach helps you optimize strains for high-level protein production. You can now design synthesis experiments that focus on the most promising candidates.
| Key Findings | Description |
|---|---|
| Prediction Accuracy | Machine learning models achieved high prediction accuracy with fewer training sequences than previously expected. |
| Data Efficiency | Controlled sequence diversity improved data efficiency, allowing for better model performance with smaller datasets. |
| Training Sequence Requirements | The number of training sequences needed for accurate predictions was found to be significantly lower than traditional expectations, suggesting a shift in approach for strain optimization. |
You use these tools to guide your synthesis strategy. You can predict which changes will boost high-level expression and yield. Machine learning helps you avoid wasted effort and focus on the most effective synthesis pathways.
Note: Machine learning makes your synthesis process smarter and more efficient. You can achieve high-level expression with less guesswork.
Novel Vectors and Regulatory Elements
You now have access to new vectors and regulatory elements that improve protein synthesis. Strong promoters and enhancers boost the expression levels and stability of recombinant proteins in mammalian cell cultures. You use these elements to achieve high-level expression and maintain protein quality.
- You can use a novel bidirectional promoter to express two divergent genes at similar levels. This is important when you need to produce proteins with multiple subunits, such as monoclonal antibodies.
- Artificial recruitment of transcription-associated complexes, like histone acetyltransferase p300, increases the efficiency of generating stable cell clones. You get high-level protein expression more quickly.
- Strong cellular enhancers, such as the Locus Control Region (LCR), help you enhance reporter gene transcription. These enhancers work best in specific cell lines, so you choose them based on your synthesis needs.
You see that these new tools let you fine-tune your synthesis process. You can achieve high-level expression in many systems. You also improve the stability and yield of your proteins. These advances make your synthesis work more reliable and productive.
Callout: Novel vectors and regulatory elements give you more control over synthesis and high-level expression. You can now produce complex proteins with greater efficiency.
Maltose-Binding Protein in Purification
MBP Fusion for Solubility
You often face challenges when you try to produce recombinant proteins. Many proteins do not fold well or stay soluble during protein production. You can solve this problem by using maltose-binding protein as a fusion partner. When you attach MBP to the N-terminus of your target protein, MBP folds first. This folding helps the passenger protein stay soluble and increases the overall yield. You see better results with N-terminal fusions than with C-terminal ones. Even small changes in the linker between MBP and the target protein can change solubility outcomes. You use MBP to help recombinant proteins fold correctly and avoid aggregation. This strategy makes protein production more reliable and efficient.
Tip: You should test different linker sequences to find the best solubility for your highly purified mbp-target proteins.
MBP in Eukaryotic Systems
You use maltose-binding protein not only in bacteria but also in eukaryotic systems. MBP helps you produce recombinant proteins in yeast, insect, and mammalian cells. You find that MBP improves the stability and expression of your target protein. You can purify proteins fused to MBP in one step using amylose columns. This method gives you 70-90% purity quickly. MBP also stabilizes and solubilizes passenger proteins, which helps you keep biological activity high. You see that MBP reduces toxicity and boosts expression levels. Many researchers choose MBP for protein production because it makes purification of proteins easier and more effective.
| Evidence Description | Key Findings |
|---|---|
| Purification of Proteins Fused to Maltose-Binding Protein | Achieves 70-90% purity in a single step using amylose columns. |
| One-step affinity purification of fusion proteins | Stabilizes and solubilizes passenger proteins, enhancing biological activity. |
| Maltose-Binding Protein | Increases solubility and stability of target proteins, alleviating toxicity and improving expression levels. |
You rely on MBP for recombinant protein production in many molecular biology labs. You can produce highly purified mbp-target proteins with less effort. MBP makes purification of proteins faster and more consistent. You see MBP as a key tool for producing recombinant proteins in both prokaryotic and eukaryotic systems.
Optimization Strategies
Expression Conditions
You can boost protein yield by adjusting key factors during expression. Lowering the temperature to between 15°C and 25°C helps proteins fold better and reduces aggregation. You should reduce inducer concentrations, such as IPTG, to improve solubility. Extending induction times, for example, 16 to 20 hours at 18°C, often leads to higher yields. You also benefit from optimizing pH and temperature to maximize cellular metabolism and protein folding. A hypothermic shift can double your productivity. Advanced media optimization, including transcriptional enhancers and fed-batch cultures, further increases yield.
| Expression Condition | Effect on Protein Yield |
|---|---|
| Lowering expression temperature | Improves solubility and reduces aggregation |
| Adjusting inducer concentration | Higher solubility with lower concentrations |
| Optimizing induction times | Longer times at lower temperatures yield more protein |
Tip: You can fine-tune these conditions to match your protein and system for the best results.
Troubleshooting Yield
You may face low protein yield during production. You should check if your expression system works correctly and if conditions are optimized. Make sure your lysis buffer and cell lysis methods are effective. Adjust temperature or use solubilizing agents to improve protein solubility. Confirm that affinity tags are accessible and the resin matches your protein. Use the right elution buffer with correct pH and eluting agent concentration. Analyze your samples for purity and consider extra purification steps if needed. Keep samples cold and add protease inhibitors to prevent protein degradation. For isotopically labeled proteins, grow cells in rich media before switching to labeled media. Supplement minimal media with rich nutrients for faster growth and higher expression. Try strains like C41(DE3) and C43(DE3) to overcome toxicity and improve plasmid stability.
- Verify expression system and conditions
- Ensure effective cell lysis
- Optimize solubility with temperature and agents
- Check affinity tag accessibility and resin compatibility
- Use correct elution buffer
- Analyze purity and consider further purification
- Prevent degradation with cold storage and inhibitors
- Use rich media and supplement minimal media
- Select strains for stability and toxicity resistance
Purification and Tag Removal
You can improve protein quality and recovery rates with new purification and tag removal techniques. Self-removing tags, such as split inteins, eliminate extra processing steps and reduce product loss. Expanded Bed Adsorption (EBA) captures proteins directly from bioreactor feedstreams, saving time and resources. Continuous microfluidic technologies let you test many variables efficiently, boosting production and lowering material use.
| Advancement Type | Description | Impact on Protein Quality and Recovery Rates |
|---|---|---|
| Self-Removing Tags | Split intein systems remove tags without extra steps. | Streamlines process, reduces product loss. |
| Expanded Bed Adsorption (EBA) | Captures product directly from feedstreams, skipping recovery steps. | Prevents loss, saves time and resources. |
| Continuous Microfluidic Technologies | Integrated systems test variables efficiently. | Boosts efficiency, reduces material use and carbon footprint. |
Note: You can combine these advancements to achieve higher purity and better recovery in your protein production workflow.
Challenges and Solutions
Misfolding and Aggregation
You often see proteins misfold or form clumps during production. These problems can lower yield and reduce quality. Many factors cause misfolding and aggregation. You may notice inefficient protein biogenesis, mutant proteins, or high concentrations of unstable units. Interruptions in the secretory pathway and environmental stress, such as high temperatures or chemical exposure, also play a role. Metabolic stress and aging can lead to oxidative damage.
You can use several strategies to solve these issues:
- Molecular chaperones like Hsp100, Hsp90, and Hsp70 help proteins fold correctly. They reduce the formation of inclusion bodies.
- Chemical additives, such as d-sorbitol and betaine, stabilize proteins by inducing osmotic stress.
- You can prevent aggregation by stabilizing the native state of proteins.
- Refolding misfolded proteins restores their function.
- Modifying proteins after translation helps prevent clumping.
Tip: Adjusting concentration, ionic strength, temperature, and pH can improve protein stability and reduce aggregation.
Host Cell Limitations
You may face limits when using host cells like E. coli for protein production. These cells sometimes struggle with translation efficiency and protein solubility. Metal ion transport can also affect protein yield. You can overcome these barriers with cis-optimization and trans-optimization. These methods boost expression and solubility. You select the right host cell and optimize its genetic makeup to improve results. You can also use chemical additives or molecular chaperones to help the host cell produce more stable proteins.
| Limitation | Solution |
|---|---|
| Translation issues | Cis-optimization |
| Solubility problems | Trans-optimization |
| Metal ion transport | Genetic engineering |
Consistency and Scale-Up
You want to produce proteins at large scale while keeping quality high. New technologies make this possible. Companies like Reploid use ReFarmUnit to produce thousands of tons of protein-rich larvae each year. Proteine Resources developed a beef substitute with a 1:1 nutritional profile, cutting costs by half. Agroloop and FreezeM built the first decoupled BSF protein facility, showing how insect farming can scale efficiently.
You see plant-based protein markets growing fast, with projections reaching $28.3 billion by 2025. Precision fermentation lets you make specific proteins with less environmental impact. AI-driven systems control fermentation, keeping product quality consistent and maximizing yield. Advanced bioreactor designs help you maintain stable protein expression and optimize microbial growth.
- Precision fermentation offers sustainable protein production.
- AI systems ensure consistent quality and high yield.
- Scalable facilities support mass production and market readiness.
Note: You can use these new methods to scale up protein production without losing quality or consistency.
Future Applications
Personalized Medicine
You see protein production techniques changing the way you approach personalized medicine. You can use fusion proteins to target specific disease pathways in each patient. Precision medicine uses complex personal data to improve health outcomes. You learn how sequence variants affect proteins and pathways. This helps you define causes and effects for health problems. You focus on protein function and interaction networks to create better health strategies. Advances in proteoformics let you design fusion proteins that match individual patient needs. You understand disease mechanisms more deeply and identify new drug targets. Fusion proteins allow you to tailor treatments for unique disease characteristics. You can deliver therapies that work best for each person.
- Precision medicine uses personal data for better health.
- Sequence variants help you understand protein pathways.
- Fusion proteins offer tailored drugs for individual patients.
- Proteoformics reveals new drug targets and disease mechanisms.
- You create fusion proteins that match patient needs.
- Protein interaction networks guide your health strategies.
- You design fusion proteins for specific disease pathways.
- Personalized protein drugs improve treatment outcomes.
Sustainable Synthesis
You want to produce fusion proteins in ways that protect the environment. Alternative proteins, such as plant-based and cultivated meat, use less land and water than traditional meat. You see lower greenhouse gas emissions and less air pollution. Water eutrophication decreases when you choose these methods. Life cycle assessments show that cultivated meat made with clean energy has big environmental advantages. You can use fusion proteins to create food products that are healthy and sustainable. You help reduce the impact of animal agriculture. Fusion proteins let you design new foods with better nutrition and lower resource use. You support a future where protein production does not harm the planet.
- Plant-based proteins use less land and water.
- Cultivated meat lowers greenhouse gas emissions.
- Fusion proteins create sustainable food products.
- You reduce air pollution and water eutrophication.
- Clean energy improves protein synthesis.
- Fusion proteins help you design nutritious foods.
- Life cycle assessments show environmental benefits.
- You support sustainable protein production.
Synthetic Biology
You use synthetic biology to build new protein production pathways. Fusion proteins play a key role in these advances. Cell-free protein synthesis technology lets you design, build, and test fusion proteins quickly. You do not need cell culture, so you speed up development. You can prototype synthetic metabolic pathways and identify bottleneck enzymes. AI models, such as Hyena, help you propose novel fusion proteins for many functions. Companies like Absci and Orbit Discovery use AI to explore protein engineering spaces that were too large before. Machine learning changes the way you design and optimize DNA. You see companies offering AI-guided services for engineering microbial strains and gene therapies. Fusion proteins let you create new solutions for medicine, industry, and research.
- Cell-free synthesis speeds up fusion protein design.
- You prototype synthetic pathways and find bottlenecks.
- AI models propose novel fusion proteins.
- Fusion proteins expand protein engineering possibilities.
- Machine learning improves DNA design and optimization.
- Companies offer AI-guided engineering services.
- Fusion proteins drive innovation in synthetic biology.
- You create new therapies and industrial solutions.
You see that new protein production techniques help you get higher yields and better quality. Efficient purification and smart expression systems make your work easier. Experts suggest you try precision fermentation, cellular agriculture, and algae-based proteins. You can use AI to discover new protein sources faster.
- Precision fermentation lowers costs and improves access.
- Cellular agriculture makes meat and seafood more affordable.
- Algae-based proteins become everyday foods.
- AI speeds up protein research.
| Impact Area | Description |
|---|---|
| Investment in Fermentation | Over US$4 billion invested in new fermentation methods. |
| Market Growth Projection | Protein market may reach US$3.5 trillion by 2050. |
| Cost Reduction Potential | Better bioprocessing can cut costs by up to 50%. |
You can shape the future of biotechnology by using these advances in your research and production.
FAQ
What is the best system for producing human therapeutic proteins?
You should use mammalian cell systems, such as CHO cells. These cells add human-like modifications to proteins. This helps you create safe and effective therapies for patients.
How can you increase protein solubility during expression?
You can lower the temperature and use fusion tags like MBP. These steps help proteins fold correctly and stay soluble. You may also add chemical chaperones to your culture.
Why do you choose cell-free protein synthesis?
You choose cell-free systems for speed and flexibility. You can produce proteins in hours. This method works well for toxic or hard-to-express proteins.
What is the main challenge in scaling up protein production?
You often face problems with consistency and quality. Large-scale systems need careful control of conditions. Automation and AI help you keep yields high and products reliable.
Tip: Always test small-scale conditions before moving to large-scale production.

